Abstract
Eleven samples of Polish Phacelia tanacetifolia Benth., three Brassica napus and one Salix spp. honeys were characterized by melissopalynology and analysis of the compositions of their volatile fractions. Headspace solid-phase microextraction coupled with gas chromatography mass spectrometry (HS-SPME/GC–MS) using PDMS/CAR/DVB fiber was used for the isolation of low-molecular weight compounds which create a volatile fraction. To differentiate and indicate the most representative unifloral samples, chemometric techniques such as principal component analysis (PCA) and hierarchical-tree clustering (HTC) were applied to the dataset of the chromatographic fingerprints. Based on the obtained results, a unique chemical fingerprint of phacelia honey was generated. This study allows us to discriminate the botanical origin of the phacelia honeys based on the GC–MS and HPTLC analysis. In case of the GC–MS analysis trans-linalool oxide, hotrienol, cis-linalool oxide and cis-epoxylinalool were identified as a predominant compound. Additionally lipophilic fractions obtained by ultrasound-assisted extraction (UAE) and solid-phase extraction (SPE) were subjected to the HPTLC analysis. It allowed the construction of a barcode-type identifier that could be used to differentiate the honey samples even without identifying the individual components of the obtained fraction.
Similar content being viewed by others
Introduction
Honey is defined as a natural sweet substance produced by honey bees (Apis mellifera) from floral nectar or honeydew. Recently, the popularity of honeys and other bee products, much like natural products in general, has contained to increase mostly because they are rich in biologically active compounds. Thus, natural food products are extremely desired by consumers due to their beneficial effects on human health. For thousands of years, bee honey has been valued worldwide as an ingredient in cosmetics, a nutritional component and a good therapeutic agent. Moreover, consumer confidence in honey is currently high since its beneficial effects have been scientifically proven [1,2,3].
Unfortunately, the quality of honeys present on the global market is frequently not satisfactory. The main reason is that the requirements set by the European Union and in Codex Alimentarius regarding honey quality are not sufficient. Different methods of honey adulteration have been observed; e.g., they are mixed with high sugar content syrups or mixed with lower quality honeys [4, 5]. Additionally, another important issue in honey falsification is the erroneous classification of their botanical or geographical origin. It is frightening that the practice of falsifying food products is not only seen in honeys. Therefore, the authentication of food products constitutes a significant challenge for the institutions responsible for ensuring food quality [6]. The botanical origin of honey is commonly determined based on pollen analysis [7] and basic physical, chemical [8,9,10] and biological [9] parameters. Melissopalynological analysis is based on the fact that each honey includes evidence of its botanical and geographical origin in the form of thousands of pollen grains from the plants from which nectar was collected. Unfortunately, scientific data show that both of these approaches possess significant limitations and are not sufficient for tracing honey adulteration [11].
Taking this into consideration, there has been increasing focus on the development of different analytical approaches for authenticating the botanical and geographical origin of honeys. These methods are considered to be the alternative or supporting methods to the routinely applied methods. The elaboration of such methods is, however, not a simple task since honey is a very complex matrix, and its composition depends on a large number of variable factors (e.g., climate, soil, harvest technology, beekeeping conditions, age of the honey and storage conditions) [10, 12]. One of the groups of compounds on which the researchers are focused is those present in the volatile fractions. These fractions are responsible for the aroma and flavor of the honeys. Of the studied types and varieties of honeys, more than 600 compounds have been identified in their volatile fractions [13]. These compounds belong to different chemical classes, with the most important compounds being classified as monoterpenes, sesquiterpenes, aldehydes, ketones and higher aliphatic alcohols. Moreover, they might originate directly from the plant nectar or be the result of enzymatic transformations. The volatile fractions are highly variable because many factors can influence the contents of volatile compounds (e.g., time and conditions of storage and harvesting and processing methods). Furthermore, all factors that influence the composition of the nectar of certain plants can also cause the chemical differences observed among honeys of the same variety [12, 14, 15].
The main objectives of the present study were the isolation and identification of unique volatile compounds from Polish phacelia honeys and the creation of a specific profile of their components, which might serve as a basis for the differentiation of these honeys. The honey samples analyzed herein were collected in different years (2014–2018) and from different regions of Poland. The studies were carried out using three different methods for the extraction of the volatiles and lipophilic compounds: headspace solid-phase microextraction coupled with gas chromatography mass spectrometry (HS-SPME/GC–MS) using PDMS/CAR/DVB fibers, ultrasound-assisted extraction (UAE) and solid-phase extraction (SPE). Based on the obtained results, a unique chemical fingerprint of phacelia honey was created. Furthermore, to study the composition of the lipophilic fractions, high-performance thin-layer chromatography (HPTLC) analysis was applied. This allowed the construction of barcode-type identifiers, which might be useful for differentiating honeys of different origins.
Materials and Methods
Honey Samples
A total of 15 honey samples originating from lacy phacelia (Phacelia tanacetifolia Benth.), rape (Brassica napus L.) and willow (Salix spp.) were used in this study. Samples were purchased between 2014 and 2018 from domestic apiaries located in different geographical regions in Poland. The apiaries confirmed the botanical origin of these honeys, and each honey sample was subjected to pollen analysis. All analyses were performed on an Olympus BX41 microscope (Olympus America, PA, USA) under 400 × magnification according to the methodology recommended by the International Commission of Bee Botany and by the International Honey Commission [16]. The results of the pollen analyses of the tested honeys are presented in Table 1 and they support the correct assignment of the honey types by the beekeepers. The honeys were stored at 4 °C and analyzed in the year of the harvesting.
Chemicals and Reagents
All chemicals used in this study were of analytical grade. Solvents (dichloromethane, methanol, ethanol, toluene, ethyl acetate, and glacial acetic acid), sodium chloride, anhydrous magnesium sulfate and 95% sulfuric acid were purchased from POCH S.A. (Gliwice, Poland). The homologous series of n-alkanes C9–C25, anisaldehyde and all compounds used as references were purchased from Sigma-Aldrich (Poznań, Poland). Strata SDB-L SPE cartridges were purchased from Phenomenex, while silica gel 60 HPTLC aluminum plates (20 cm × 10 cm) were purchased from Merck.
Extraction and Determination of the Volatile Compounds
Headspace Solid-Phase Microextraction (HS-SPME) Methodology
The composition of the volatile fractions of eleven phacelia, three rape and one willow honeys were determined using HS-SPME/GC–MS. Each honey sample was analyzed in triplicate. Moreover, to control and avoid overloading the SPME fiber, blank analyses were run between sequential analyses. Before the experiments, the fiber was conditioned according to the manufacturer’s instructions. The details of the analysis procedure were described previously [17]. For analysis, 5 g of honey was dissolved in 5 mL of distilled water, and 0.75 g of NaCl was added. The mixture was placed in a 15 mL vial, which was then tightly sealed with a PTFE–silicone septum. The extraction was performed in a thermostatic bath at 70 ± 1 °C under constant agitation for 45 min (including 15 min of preincubation). The extracted volatiles were thermally desorbed in the injection port of the GC–MS for 5 min at 250 °C.
GC–MS
An Agilent 6890 GC in combination with a 5973 MSD was used. Separation was done on a 30 m × 0.25 mm ID × 0.25 µm HP-5MS column (Agilent Technologies). The mass spectrometer was operated in the SCAN mode (m/z: 35–350). Other analytical conditions were used as described previously [16]. Benzophenone (with a final concentration of 10 ppm) was used as an internal standard. The average percentage of each component was calculated using the normalization method (without a correction factor) based on the area of the GC peaks. The compounds were identified based on a comparison of their spectra with those deposited in the NIST 14 library (National Institute of Standards and Technology, Gaithersburg, MD, USA) or with standard substances available in our laboratory. Moreover, the retention indices calculated for each peak relative to C9–C25n-alkanes on the HP-5 MS column were compared with those present in the literature (NIST Chemistry WebBook, NIST Standard Reference Database Number 69, http://www.webbook.nist.gov/chemistry/). Each analysis was performed in triplicate.
Extraction and Determination of the Lipophilic Fraction
Ultrasound-Assisted Extraction (UAE) and Solid-Phase Extraction (SPE)
Ultrasound solvent extraction and solid-phase extraction were performed according to the procedures described previously [17,18,19]. For each honey sample, the extraction was performed in triplicate. To obtain the UAE extracts, 40 g of honey was dissolved in 20 mL of distilled water, and 1.6 g of anhydrous magnesium sulfate was added. The aqueous solution was then extracted three times with 22-mL portions of dichloromethane using an ultrasonic cleaning bath (Cole-Parmer 8891) at 25 °C for 30 min. After each sonication step, the whole extract was centrifuged at 3000 rpm. The collected organic layers were combined and dried over anhydrous magnesium sulfate. In the case of SPE, the entire procedure was carried out in a Baker SPE-12G vacuum manifold (J. T. Baker®, Phillipsburg, NJ, USA) at a flow rate of 1.5 mL/min using 3-mL Strata SDB-L cartridges with 200 mg of styrene–divinylbenzene resin (Phenomenex). Prior to use, the cartridges were conditioned by rinsing sequentially with 6 mL of dichloromethane, water and an ethanol–water mixture (12%, v/v). Ten grams of each honey sample was dissolved in 30 mL of distilled water, filtered through filter paper and passed through a cartridge. Subsequently, the hydrophilic components and the remaining sugars were flushed out with 20 mL of water, and finally, the volatile and semivolatile compounds were eluted with 10 mL of dichloromethane. The organic fractions were dried over anhydrous magnesium sulfate. The resulting extracts after both extraction procedures were concentrated on a rotary evaporator at 35 °C, and the obtained oily residues were dissolved in 350 µL of dichloromethane and stored at 4 °C until HPTLC analysis.
HPTLC Separation
High-performance thin-layer chromatography was used to analyze the lipophilic fractions obtained by UAE and SPE using the parameters developed in our laboratory [17, 19]. Ten microliters of each extract at a concentration of 100 ± 5 ng/spot was applied to the plate in 10 mm bands 15 mm from the lower edge of the plate using a semiautomated HPTLC application device (Linomat 5, CAMAG, Muttenz, Switzerland) under a constant flow of nitrogen gas and at a rate of 250 nL·s−1. Precoated silica gel 60 HPTLC aluminum plates (20 × 10 cm) were used. The separations were performed in a chromatographic tank, which was saturated with the mobile phase (toluene-ethyl acetate [80:20 (v/v)]) for 20 min before analysis. The spots were developed to a distance of 90 mm. Then, the plates were visualized using an HPTLC imaging device (TLC Visualizer, CAMAG) and examined by visionCATS and CAMAG software under white light and with irradiation at 254 nm and 366 nm. At the final stage, the plates were derivatized with anisaldehyde, dried at 105 °C for 5 min and visualized again.
Statistical Analysis
Statistical analyses were performed using STATISTICA 13.1 software (StatSoft Inc., Tulsa, OK, USA). PermutMatrix software was used for heatmap creation [19]. The obtained data were analyzed via hierarchical cluster analysis (complete linkage using Euclidean distance) and principal component analysis (PCA). These two classification techniques were used to discover the natural groupings of the data and examine the variations between the analyzed honey samples.
Results and Discussion
Phacelia honey is produced from the nectar of lacy phacelia (Phacelia tanacetifolia Benth.), which belongs to the Boraginaceae family and it native to Texas. Despite the fact that phacelia plants are considered one of the greatest nectar-providing plants for bees [20, 21], phacelia honey is still one of the least popular unifloral honeys in the world. The ability to provide food for bees even under extreme weather conditions is one of its greatest advantages [21]. Monofloral phacelia honeys are light honeys (the color is characterized as pale, amber, light green, reddish-brown or even white). Furthermore, it is characterized by delicate flavor and aroma [22,23,24]. Compared to other unifloral honeys, characterization data for phacelia are scarce, and only a few papers have reported their physical–chemical parameters [25,26,27,28], total flavonoid and phenolic contents [29, 30] and the composition of their volatile fractions [27, 31]. Moreover, taking into account that phacelia pollen may be overrepresented over other types of pollen [24, 32], it is worth expanding the available data to avoid the mislabeling of honeys. Therefore, the main purpose of this study was to isolate the volatile compounds in phacelia honeys and determine whether the generated chemical fingerprints can be used to determine their botanical origins. To create these chemical fingerprints, we use two different approaches. The first relied on headspace solid-phase microextraction (HS-SPME) with DVB/CAR/PDMS [[divinylbenzene/carboxen/polydimethylsiloxane)] for the extraction of the volatiles followed by GC–MS analysis and then application of two chemometric tools (PCA and HCA). Two methods of extraction (SPE and UAE) were applied to differentiate the phacelia honeys based on the lipophilic fraction. Eleven phacelia honey samples were collected from different locations in Poland, and their botanical origin was confirmed by pollen analysis (Table 1). Four of the samples had phacelia pollen grain contents higher than 80%, one had a content higher than 70%, four had contents higher than 60% and only two had pollen contents lower than 40%. Pollen analysis indicated that phacelia honeys are mostly contaminated with Brassica napus L. and Salix spp. Three honeys samples of the former and one sample of the latter (with well-confirmed botanical origins) were included in this study.
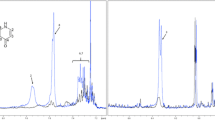
GC–MS analysis of the obtained extracts allowed the identification of 79 volatile compounds belonging to various chemical classes. Exemplary TIC chromatograms are presented in Fig. 1, and all the obtained results are presented in Table 2.
In the analysis of the obtained results, several isolated fatty acids, their esters, and higher nonpolar hydrocarbons, such as n-hexadecanoic acid, nonadecane, tricosane, 1-docosene, (Z)-octadec-9-enal, and (Z,Z)-9,12-octadecadienoic acid, were excluded since these compounds are widespread among honeys of many origins and are not indicative of the botanical source. Among the isolated chemical constitutes of pure phacelia honeys, the most abundant compounds that were present in all the samples were trans-linalool oxide (14.1–56.0%), hotrienol (2.7–14.7%), cis-linalool oxide (5.8–10.1%) and cis-epoxylinalool (0.8–2.5%). The pure rape honeys showed sulfides, which are characteristics of Brassicaceae honeys [33]; specifically, they showed higher amounts of dimethyl disulfide (12.8–17.7%). Moreover, the same compounds were also determined in the FA-8 sample, and although it was labeled as phacelia, rape pollen was dominant. Additionally, in honeys in which Salix spp. or Brassica napus L. were the dominant pollens, compounds such as safranal, trans-cinnamaldehyde, 4-vinylguaiacol, p-cymenene, p-cymene and p-cymen-8-ol, 4-oxoisophrone and 3-hydroxy-β-damascone, methyl syringate, limonene-1,2-diol and 1,3,8-p-menthatriene were observed, and these compounds were previously identified in willow and rape honeys [34,35,, 35]. Interestingly Špánik et al. (2012) indicated that 1,3,8-p-menthatriene is unique to phacelia honeys [27], whereas in our study, as well as the report published by Kuś et al. [28], this compound was not detected in pure phacelia honeys. Our results suggest that the presence of 1,3,8-p-menthatriene is more likely correlated with the abundance of Salix spp. pollen grains. The obtained data strongly indicate that linalool derivatives are common in phacelia honeys. They are present in a significant percentage of honeys in which the phacelia pollen content is higher than 60%, whereas in samples with lower amounts of phacelia pollen, these compounds are observed less frequently. These data also correlate well with those presented in the literature [28, 31]. Thus, Kuś et al. analyzed samples of Polish phacelia honeys and indicated a high content of linalool derivatives and postulated that these compounds could be considered potential markers [28].
The identified volatiles were used to create the heatmap shown in Fig. 2. It is a simple matrix that represents the relationships between the percentages of each identified compound and the corresponding honey samples. The heatmap allow the fast differentiation of different honey varieties based on the resultant code system. The growing literature data on the analysis of the chemical compositions of monofloral honeys clearly indicate that it is practically impossible to identify single specific markers for each of type of honey. Various honeys are often characterized by the presence of the same markers, and the use of libraries of their chemical components is reasonable for their precise discrimination. Heatmaps, which provide a clear visual representation of obtained data, could be a very useful tool in such cases.
Since the studied phacelia honeys are of variable botanical purity, principal component analysis (PCA) and hierarchical clustering analysis (HCA) were conducted to sort the honey samples. The obtained results are presented in Fig. 3(a, b). In the case of PCA, the first two components explained 75.2% of the data variance (PC1 explained 56.55% of the mentioned variance, whereas PC2 explained 18.76%). Although PCA is classified as an unsupervised method, it gave satisfactory results and allowed us to discriminate the honeys because some natural groupings of the samples were observed in the PCA score plot; three main groups were observed. The first group corresponds to pure rape honey samples. The second includes phacelia honeys of various purities (FA-2, FA-3, FA-5, FA-6, FA-7, FA-9, FA-10 and FA-11). Sample FA-8, in which only 39.5% of the pollen present is phacelia and 40.3% is rape pollen is still more correlated with phacelia than with the rape honeys. In contrast, FA-1, which contains 38.9% phacelia honey and 32.3% Salix pollen, was more correlated with willow honey. These two honey samples, along with FA-4, form the third group in the PCA plot. FA-4 contained 60.5% phacelia pollen with the remaining pollens being present in minute quantities. Its chemical composition evidently does not fit into the standards of phacelia honeys. We speculate that additional pollen may have been added.
Among the studied compounds, the percentage of some poses either significantly negative or positive contributions to PC1 and PC2 (e.g., the abundances of trans-linalool oxide, cis-linalool oxide, hotrienol, and phenylacetaldehyde exhibit significant negative contributions to PC1, whereas the abundances of dimethyl sulfide, dimethyl disulfide, dimethyl trisulfide, phenylethyl alcohol, and nonanoic acid exhibit significant positive contributions to PC2). If PC3 is included in the other analyses, a total of 88.3% of the variance in the data is explained. Significant negative contributions from p-cymenene, p-cymen-8-ol, safranal and trans-cinnamaldehyde was observed for this factor.
The dendrogram of the chemical profiles obtained by HCA (complete linkage using Euclidean distances) presented in Fig. 3b shows that all the honey samples clustered into five groups based on their pollen composition. The first group contains samples in which the amount of Phacelia tanacetifolia Benth. is between 76 and 85%, and the second group includes honeys with phacelia pollen contents in the range of 40–69%. Honeys classified in both of these groups can be considered pure phacelia honeys.
According to the pollen analysis, sample FA-4 contains 60.5% phacelia pollen grains; however, in both PCA and HCA, it is naturally grouped with FA-1 and Wi-1. Sample FA-1 can be considered mixed phacelia–Salix, whereas sample F-4 is characterized by a high phacelia pollen content and should fit into the second group. Additionally, the same trend is observed in the heatmap analysis; FA-4 clearly exhibits similarities with FA-1 and Wi-1 but not with pure phacelia honeys. This observation confirmed the suggestion that this sample could have been falsified by the addition of pollen after harvesting, and its actual origin may be different from that found by melissopalynological analysis.
In summary, our results suggest that to recognize honey as pure phacelia, the content of phacelia pollen should be higher than 50–60%. Kuś et al. [28] reached the same conclusion from their studies.
HPTLC Analysis
To obtain specific HPTLC fingerprints for phacelia honeys, UAE and SPE extracts were obtained. The dominant extracted compounds in the volatile fraction determined by HS-SPME were assessed by HPTLC. The idea of the supplementation of HS-SPME studies with the determination of the compositions of extracts was described previously [19]. The obtained bands on the HPTLC plates, which are presented in Fig. 4(a–d) and in Fig. 1(a, b) in supplementary materials, create specific and unique fingerprints that are reminiscent of commercially used barcodes. The most characteristic zones and their colors are presented in Table 3. Slight differences were observed among the results obtained from the two extraction methods. The most characteristic feature of the UAE extracts is the presence of deep gray (white light) or black (irradiation at 254 nm and 366 nm) zones at Rf = 0.10. Furthermore, based on a visual comparison of the obtained results, it is possible to observe the differences between samples FA-1 and FA-4 and the rest of the studied samples. It seems that compared to typical approaches for the differentiation of honeys of different botanical origins, which are based on the analysis of the chemical composition of their volatile fractions, HPTLC could be considered a method of choice because it allows accurate and fast visual discrimination of honey samples. This technique can be particularly useful as a fast screening method because of its high precision and low time consumption. Moreover, as it was described previously and was confirmed in this study [18] the UAE extraction is probably more suitable for lipophilic fraction profile creation seeing that the extracted compounds are present in higher concentration than in SPE fraction and for screening purposes one cycle of extraction is suitable, so the time and cost are decreasing. Furthermore, the number of steps needed to obtained the fraction ready to analysis is fewer than in case of SPE.
Conclusion
In the present study, the profiling of the volatile fractions of Polish phacelia (Phacelia tanacetifolia Benth) honey samples was performed using two different approaches: HS-SPME/GC–MS and HPTLC analysis of the extracts acquired by UAE and SPE. Melissopalynological analysis indicated that the purchased phacelia honeys contained significant amounts of rape and Salix pollen. Therefore, pure rape and Salix honeys were also studied in the same manner for comparison. The HS-SPME/GC–MS method indicated linalool derivatives could be used as markers for botanical phacelia-derived honeys. These studies were supported by HPTLC analysis of the extracts obtained by means of UAE and SPE. The results obtained by these two methods are presented visually as graphs reminiscent of commercially used barcodes (in the case of HS-SPME/GC–MS, a heatmap was constructed, and in the case of HPTLC, each sample applied on the plate created a unique pattern). Our studies seem to prove that the idea of generating such fingerprints based on the identification of libraries of compounds (based on GC–MS analysis or only based on the Rf values and band colors visible by HPTLC) may be a good alternative for routine pollen analysis.
References
Bodganov S, Jurendic T, Sieber R, Gallmann P (2008) J Agric Food Chem 276:677–689
Nagai T, Inoue R, Kanamori N, Suzuki N, Nagashima T (2006) Food Chem 97:256–262
Ajibola A, Chamunorwa JP, Erlwanger KH (2012) Nutr Metab 9(1):12
Mato I, Huidobro JF, Simal-Lozano JS, Sancho MT (2006) J Agric Food Chem 54:1541–1550
Martos I, Cossentini M, Ferreres F, Tomás-Barberá FA (1997) J Agric Food Chem 45:2824–2829
Pustjens AM, Muilwijk M, Weesepoel Y, van Ruth SM (2016) In: Downey G (ed) Advances in authenticity testing of geographical origin of food products, 1st edn. Woodhead Publishing, Sawston, Cambridage
Louveaux J, Maurizio A, Vorwohl G (1978) Methods of melissopalynology. Bee World 59(4):139–157
Anklam E (1998) Food Chem 63:549–562
Popek S (2002) Food Chem 79:401–406
Kaskoniene V, Venskutoni PR (2010) Compr Rev Food Sci Food Saf 9:620–635
Bogdanov S, Martin P (2002) Mitt Geb Lebensmittelunters Hyg 93:232–254
da Silva PM, Gauche C, Valdemiro L, Costa ACO, Fett R (2016) Food Chem 196:309–323
Manyi-Loh C, Ndip RN, Clarke AM (2011) Int J Mol Sci 12:9514–9532
Ouchemoukh S, Schweitzer P, Bachir Bey M, Djoudad-Kadji H, Louaileche H (2010) Food Chem 121:561–568
Siddiqui AJ, Musharraf SM, Iqbal Choudhary M, Rahman A (2017) Food Chem 217:687–698
Makowicz E, Kafarski P, Jasicka-Misiak I (2018) Eur Food Res Technol 244:2169–2179
Jasicka-Misiak I, Makowicz E, Stanek N (2018) Eur Food Res Technol 244:1169–1184
Makowicz E, Jasicka-Misiak I, Teper D, Kafarski P (2018) Molecules 23:1811
Caraux G, Pinloche S (2005) Bioinformatics 21:1280–1281
Farkas Á, Zajácz E (2007) Eur J Plant Sci Biotechnol 1:125–151
Trzybiński S (2010) Miód faceliowy i inne miody cz 2. Pasieka. https://pasieka24.pl. Accessed 2 Sept 2018
Petrus K, Schwartz H, Sontag G (2011) Anal Bioanal Chem 400(8):2555–2563
von der Ohe W (2005) Niedersächsisches Landesinstitut für Bienenkunde, Die Farbe des Honigs. https://www.laves.niedersachsen.de/download/41103/Die_Farbe_des_Honigs.pdf. Accessed 2 Sept 2018
Kuś PM, Jerković I, Marijanović Z, Kranjac M, Tuberoso CIG (2018) Food Res Int 106:243–253
Persano Oddo L, Piro R, Czech PI, Lamini CF, Heritier JL, France MMO, Bogdanov SB (2004) Main European unifloral honeys: descriptive sheets. Apidologie 1(35):38–81
Manikis I, Thrasivoulou A (2001) Apiacta 35(4):230–236
von der Ohe W, von der Ohe K (1999) Niedersächsisches Landesinstitut für Bienenkunde, Phaceliahonig. https://www.laves.niedersachsen.de/download/40995/Phaceliahonig.pdf. Accessed 2 Sept 2018
Dimins F, Kuka P, Augspole, I (2009) International conference on food innovation. Food Innova 2010, Valencia, pp 5–8
Petrus K, Schwartz H, Sontag G (2011) Anal Bioanal Chem 400(8):2555–2563
Špánik I, Janáčová A, Šusterová Z, Jakubík T, Jánošková N, Novák P, Chlebo R (2012) Chem Pap 67(2):127–134
Bryant VM, Jones GD (2001) Palynology 25:11–28
Radovic BS, Careri M, Mangia A, Musci M, Gerboles M, Anklam E (2001) Food Chem 72(4):511–520
Jerković I, Marijanović Z (2010) Chem Biodivers 7:2309–2325
Jerković I, Kuś PM, Tuberoso CI, Šarolić M (2014) Food Chem 15:8–14
Ruisinger B, Schieberle P (2012) J Agric Food Chem 60(17):4186–4194
Acknowledgements
This research was supported by National Science Centre, Poland Projects 2017/25/N/NZ9/00623 and 2014/2015/15/B/NZ9/02182.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Published in the topical collection The 41st Symposium Chromatographic Methods of Investigating Organic Compounds with guest editor Michał Daszykowski.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Makowicz, E., Jasicka-Misiak, I., Teper, D. et al. Botanical Origin Authentication of Polish Phacelia Honey Using the Combination of Volatile Fraction Profiling by HS-SPME and Lipophilic Fraction Profiling by HPTLC. Chromatographia 82, 1541–1553 (2019). https://doi.org/10.1007/s10337-019-03778-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10337-019-03778-x