Abstract
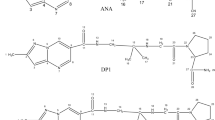
Cabergoline is widely used as a prolactin secretion inhibitor and as a treatment for Parkinson’s disease. Studying the structure of cabergoline, it contains a urea moiety and an amide group which are sensitive to degradation by hydrolysis as well as an alkene bond that is susceptible to oxidation. Degradation was performed regarding ICH recommendations including hydrolysis (pH from 1.3 to 12.7 using HCl or NaOH with different molarities), oxidation, photo, and thermal degradations. The drug was highly sensitive to all studied conditions except thermal degradation with the production of three major degradation products which were isolated and identified using IR and MS analyses. Two stability indicating chromatographic methods were developed for quantification of the drug in the presence of its degradation products. The first method was HPTLC which depended on using a developing system of butanol:methanol:triethyl amine (95:5:10, by volume) and UV scanning at 280 nm. The second method was HPLC at which the drug and degradation products were separated within 5 min using acetonitrile: 0.05% aqueous triethylamine (TEA) (pH adjusted to 6.5 using 1% aqueous H3PO4) [70:30, v/v] and UV scanning was performed at 225 nm. Validation parameters were calculated according to ICH recommendations and all parameters were within the acceptable limits. Comparison was made between the developed methods and the previously published ones; the developed methods were found to be superior regarding analysis time, studying different degradation pathways, and identifying the degradation products.





Similar content being viewed by others
References
British Pharmacopoeia (2012) Her Majesty’s stationary office. Electronic Version, London
The United States Pharmacopeia (2012) National formulary 35, 30th edn. United States Pharmacopeia Convention Inc., New York
Budavari S (2006) The Merck index, 14th edn. Merck and Co Inc., Whitehouse Station
Martindale-Extra Pharmacopoeia (2005) The complete drug references, 34th edn. The pharmaceutical Press, London
Salman D, Dogan A, Basci NE (2011) Spectrophotometric analysis of cabergoline in pharmaceutical preparations. Latin Am J Pharm 30:304–310
Onal A, Cağlar S (2007) Spectrophotometric determination of dopaminergic drugs used for Parkinson’s disease, cabergoline and ropinirole in pharmaceutical preparations. Chem Pharm Bull 55(4):629–631
Rambabu C, Jyothirmayee CA, Raju KN (2012) Spectrophotometric determination of cabergoline in tablet dosage forms. Int J Pharm Chem Biolog Sci (IJPCBS) 2(1):72–77
Rambabu C, Jyothirmayee CA, Raju KN (2012) Spectrophotometric determination of cabergoline in tablet dosage forms. Asian J Res Chem 5(2):279–281
Rambabu C, Jyothirmayee CA, Raju KN (2012) Spectrophotometric determination of cabergoline in tablet dosage form. Int J Pharm Chem Biol Sci 2(1):72–77
Jyothirmayee CA, Raju KN (2014) RP-HPLC method for the estimation of cabergoline in pharmaceutical formulations. Int J Res Pharm Chem 4(4):977–981
Magnes C, Suppan M, Thomas R (2008) Validated comprehensive analytical method for quantification of cabergoline by LC/MS/MS. Anal Chem 80(15):5736–5742
Kimball BA, DeLiberto TJ, Johnston JJ (2001) Determination of cabergoline by electrospray ionization tandem mass spectrometry: picogram detection via column focusing sample introduction. Anal Chem 73:4972–4976
Lasan VM, Indrekar TD (2016) Development and validation of stability indicating analytical method for the determination of cabergoline. Int J Pharm Res Sch (IJPRS) 5(1–2):141–150
Ӧnal A, Sagırlı O, Sensory D (2007) Selective LC determination of cabergoline in the bulk drug and in tablets: in vitro dissolution studies. Chromatographia 65:561–567
Athak AP, Rajput SJ (2009) Development of a stability indicating HPLC method for simultaneous determination of olanzapine and fluoxetine in combined dosage forms. J Chromatogr Sci 47:605–611
Darwish IA, Askal HF, Khedr AS, Mahmoud RM (2008) Stability indicating TLC method for quantitative determination of rebaverine. J Chromatogr Sci 46:5–11
Abiramasundair A, Joshi RP, Jalani HB, Sharma JA, Pandya DH, Pandya AN, Sudarsanam V, Vasu KK (2014) Stability-indicating assay method for determination of actarit, its process related impurities and degradation products: insight into stability profile and degradation pathways. J Pharm Anal 4:374–383
Abdelwahab NS, Farid NF (2014) Validated HPLC-DAD method for stability study of sulbutiamine HCl. RSC Adv 4:30523–30529
Sreenivasa PR, Uttam KR, Hiriyanna SG, Sumathi VR, Mukkanti K (2011) Identification of oxidative degradation impurities of olanzapine drug substance as well as drug product. J Pharm Biomed Anal 56:413–418
Sabry SM, Belal TS, Barary MH, Ibrahim MEA (2013) A validated HPLC method for the simultaneous determination of naftidrofuryl oxalate and its degradation product (metabolite), naftidrofuryl acid: applications to pharmaceutical tablets and biological samples. Drug Test Anal 5:500–508
Jafari-Nodoushan M, Barzin J, Mobedi H (2016) A stability-indicating HPLC method for simultaneous determination of morphine and naltrexone. J Chromatogr B 1011:163–170
Emam AA (2018) Canagliflozin stability study and ecofriendly chromatographic determination of its degradation product: a comparative study. J Sep Sci 41:822–830
ICH, Harmonized Tripartite Guidelines (2003) Stability testing of new drug substances and products. Q1A (R2): International conference on harmonization, IFPMA, Geneva, pp 1–18
Through website: http://www.toxipedia.org/display/toxipedia/Chloroform. Accessed 29 Dec 2018
Through website: https://owlcation.com/stem/Chloroform-in-the-Environment-and-its-Dangers. Accessed 29 Dec 2018
Sarmah AK, Sabadie J (2002) Hydrolysis of sulfonylurea herbicides in soils and aqueous solutions: a review. J Agric Food Chem 50:6253–6265
Bansal G, Singh M, Jindal KC, Singh S (2008) LC and LC-MS study on establishment of degradation pathway of glipizide under forced decomposition conditions. J Chromatogr Sci 46:510–517
Carl RK, Mark JL (2007) Amide resonance correlates with a breadth of C–N rotation barriers. J Am Chem Soc 129(9):2521–2528
Kovaříková P, Klimeš J, Dohnal J, Tisovská L (2004) HPLC study of glimepiride under hydrolytic stress conditions. J Pharm Biomed Anal 36:205–209
Abdelwahab NS, Elsaady MT, Korany AG, Hegazy MA (2017) Study of gliquidone degradation behavior by high performance thin-layer chromatography and ultra performance liquid chromatography methods. Biomed Chromatogr 31:4025–4035
Through website: https://chem.libretexts.org/Courses/Sacramento_City_College/SCC%3A_Chem_420_Organic_Chemistry_I/Text/09%3A_Reactions_of_Alkenes/9.12%3A_Oxidation_of_Alkenes_-_Epoxidation. Accessed 22 May 2019
International Conference on Harmonisation (ICH) (2005) Validation of analytical procedures: text and methodology Q2(R1). ICH, pp 1–13
Srivastava MM (2011) High performance thin layer chromatography (HPTLC). Springer, Heidelberg, pp 33–38
Acknowledgements
The authors express their appreciation and would like to thank Dr. Asmaa Mohamed Aboul Magd, Pharmaceutical Chemistry Department, Nahda University (NUB), Beni-Suef, Egypt, for his effort in elucidation of the chemical structure of the degradation products.
Funding
This work is not funded.
Author information
Authors and Affiliations
Contributions
Both Dr. NFF and Dr. NSA did the practical work and wrote the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Nehal Fayek Farid declares that she has no conflict of interest. Nada Sayed Abdelwahab declares that she has no conflict of interest.
Research involving human participants and/or animals
No human volunteers or animals were used in this work.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Farid, N.F., Abdelwahab, N.S. Development and Validation of Different Chromatographic Methods for Analysis of Cabergoline in the Presence of Its Degradation Products: Studying Degradation Profile. Chromatographia 82, 1555–1569 (2019). https://doi.org/10.1007/s10337-019-03763-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10337-019-03763-4