Abstract
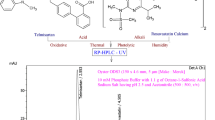
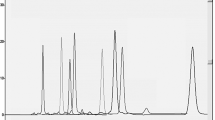
Combined drug therapy which is based on the co-administration of two or more drugs, have been used for a long time, to treat diseases. In this study, a simple, selective and rapid RP-LC method has been developed and validated for the sensitive and simultaneous determination of simvastatin (SMV) and ezetimibe (EZE) in fixed-dose combination tablets and in rabbit serum using a simple sample preparation procedure. The developed RPLC method for these lipid-lowering agents was completely validated and in the linear range of 0.05–50 µg mL− 1 EZE and 0.05–10 µg mL− 1 SMV. The calibration curves were obtained with limit of detection values of 0.013 µg mL− 1, 0.009 µg mL− 1—for EZE and SMV, respectively. The developed method was successfully applied to the analysis of EZE and SMV in fixed-dose combination tablets and in rabbit serum, and no interference was observed from any excipients and endogenous substances in the rabbit serum samples. Dissolution profiles of the pharmaceutical dosage form of these lipid-lowering agents were also studied.


Similar content being viewed by others
References
Li MC, Whitmore WF, Golbey R, Grabstald H (1960) Effects of combined drug therapy on metastatic cancer of the testis. JAMA 174:1291. https://doi.org/10.1001/jama.1960.03030100059013
Casey JF, Hollister LE, Klett CJ et al (1961) Combined drug therapy of chronic schizophrenics. Controlled evaluation of placebo, dextro-amphetamine, imipiramine, isocarboxazid and trifluoperazine added to maintenance doses of chlorpromazine. Am J Psychiatry 117:997–1003. https://doi.org/10.1176/ajp.117.11.997
Chou T-C (2006) Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol Rev 58:621–681. https://doi.org/10.1124/pr.58.3.10
Chou TC (2010) Drug combination studies and their synergy quantification using the chou-talalay method. Cancer Res 70:440–446
Goldin A, Mantel N (1957) The employment of combinations of drugs in the chemotherapy of neoplasia: a review. Cancer Res 17:635–654. https://doi.org/10.1158/0008-5472.can-09-1947
Roth A, Maibach R, Martinelli G et al (2000) Docetaxel (Taxotere®)-cisplatin (TC): an effective drug combination in gastric carcinoma. Ann Oncol 11(3):301-306 (academic.oup.com)
Demirbolat G, Altintas L, Yilmaz S et al (2018) Development of orally applicable, combinatorial drug–loaded nanoparticles for the treatment of fibrosarcoma. J Pharm Sci 107(5):1398–1407
Kalra S, Kalra B, Agrawal N (2010) Combination therapy in hypertension: an update. Diabetol Metab Syndr 2:44. https://doi.org/10.1186/1758-5996-2-44
George F, Barbara, Speed C et al (2011) Managing hypertension using combination therapy. Am Fam Phys 83:1207–1208
Nutescu EA, Shapiro NL (2003) Ezetimibe: a selective cholesterol absorption inhibitor. Pharmacotherapy 23:1463–1474. https://doi.org/10.1592/phco.23.14.1463.31942
Dujovne CA, Ettinger MP, McNeer JF et al (2002) Efficacy and safety of a potent new selective cholesterol absorption inhibitor, ezetimibe, in patients with primary hypercholesterolemia. Am J Cardiol 90:1092–1097. https://doi.org/10.1016/S0002-9149(02)02798-4
Bays HE, Moore PB, Drehobl MA et al (2001) Effectiveness and tolerability of ezetimibe in patients with primary hypercholesterolemia: pooled analysis of two phase II studies. Clin Ther 23:1209–1230. https://doi.org/10.1016/S0149-2918(01)80102-8
Ballantyne CM, Houri J, Notarbartolo A et al (2003) Effect of ezetimibe coadministered with atorvastatin in 628 patients with primary hypercholesterolemia: a prospective, randomized, double-blind trial. Circulation 107:2409–2415. https://doi.org/10.1161/01.CIR.0000068312.21969.C8
Davidson MH, McGarry T, Bettis R et al (2002) Ezetimibe coadministered with simvastatin in patients with primary hypercholesterolemia. J Am Coll Cardiol 40:2125–2134. https://doi.org/10.1016/S0735-1097(02)02610-4
Kastelein JJP, Akdim F, Stroes ESG et al (2008) Simvastatin with or without ezetimibe in familial hypercholesterolemia. N Engl J Med 358:1431–1443. https://doi.org/10.1056/NEJMoa0800742
Naidu KR, Kale UN, Shingare MS (2005) Stability indicating RP-HPLC method for simultaneous determination of amlodipine and benazepril hydrochloride from their combination drug product. J Pharm Biomed Anal 39:147–155. https://doi.org/10.1016/J.JPBA.2005.04.001
Kurbanoglu S, Gumustas M, Ozkan SA (2013) Simultaneous estimation and validation of some binary mixtures of antihypertensive drugs by RP-LC methods using two new generation silica columns. J Pharm Biomed Anal 72:198–201. https://doi.org/10.1016/J.JPBA.2012.08.018
Ozkan CK, Kurbanoglu S, Esim O et al (2016) Simultaneous determination and drug dissolution testing of combined amlodipine tablet formulations using RP-LC. Chromatographia 79:1143–1151. https://doi.org/10.1007/s10337-016-3125-x
Kurbanoglu S, Gumustas M, Uslu B, Ozkan SA (2013) A sensitive and selective RP-LC method for the simultaneous determination of the antihypertensive drugs, enalapril, lercanidipine, nitrendipine and their validation. Chromatographia 76:1477–1485. https://doi.org/10.1007/s10337-013-2465-z
Ashfaq M, Ullahkhan I, Qutab SS, Naeemrazzaq S (2007) HPLC determination of ezetimibe and simvastatin in pharmaceutical formulations. J Chil Chem Soc 52:1220–1223. https://doi.org/10.4067/S0717-97072007000300005
Wang Y, Liang Y, Zhang J et al (2016) Simultaneous separation and determination of four main isoflavonoids in Astragali Radix by an isocratic LC/ESI-MS method. J Cent South Univ 23:303–309. https://doi.org/10.1007/s11771-016-3074-4
Palabiyik IM, Onur F, Yardimci C, Özaltin N (2008) Simultaneous spectrophotometric determination of ezetimibe and simvastatin in pharmaceutical preparations using chemometric techniques. Quim Nova 31:1121–1124. https://doi.org/10.1590/s0100-40422008000500035
Hefnawy M, Al-Omar M, Julkhuf S (2009) Rapid and sensitive simultaneous determination of ezetimibe and simvastatin from their combination drug products by monolithic silica high-performance liquid chromatographic column. J Pharm Biomed Anal 50:527–534. https://doi.org/10.1016/J.JPBA.2009.05.002
The United States Pharmacopeia (The USP 40-NF 35) (2017) The official compendia of standards. United States Pharmacopeial Convention, MD, Rockwille, USA
Michael E. Swartz ISK (1997) Analytical method development and validation. J Pharm Res 3:96
Chan CC, Lam. H, Lee YC, Zhang X-M (2004) Analytical method validation and instrument performance verification. Wiley, New York
ICH Guidelines T (1995) Validation of analytical procedures, methodology international council on harmonization. Brussels, Belgium
Swartz ME, Krull IS (1997) Analytical method development and validation, vol 3. Marcel Dekker, New York, p 96
Mendyk A, Jachowicz R (2007) Unified methodology of neural analysis in decision support systems built for pharmaceutical technology. Expert Syst Appl 32:1124–1131. https://doi.org/10.1016/J.ESWA.2006.02.019
Taupitz T, Dressman JB, Klein S (2013) New formulation approaches to improve solubility and drug release from fixed dose combinations: case examples pioglitazone/glimepiride and ezetimibe/simvastatin. Eur J Pharm Biopharm 84:208–218. https://doi.org/10.1016/J.EJPB.2012.11.027
Costa P, Sousa Lobo JM (2001) Modeling and comparison of dissolution profiles. Eur J Pharm Sci 13:123–133. https://doi.org/10.1016/S0928-0987(01)00095-1
Acknowledgements
This paper is dedicated to Chromatographia 50th Anniversary commemorative issue.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
All authors declare that he or she has no conflict of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Additional information
Published in Chromatographia’s 50th Anniversary Commemorative Issue.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Figure SI. 1
. Calibration graphs for EZE and SMV in A-C mobile phase and B-D rabbit serum (PDF 51 KB)
Rights and permissions
About this article
Cite this article
Kurbanoglu, S., Esim, O., Ozkan, C.K. et al. Development and Validation of RP-LC Method for the Simultaneous Determination of Simvastatin and Ezetimibe in Fixed-Dose Combination Tablets and in Rabbit Serum. Chromatographia 82, 279–285 (2019). https://doi.org/10.1007/s10337-018-3642-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10337-018-3642-x