Abstract
Development of efficient methods for isolation and separation of biologically active compounds remains an important challenge for researchers. Designing systems such as organomineral composite materials that allow extraction of a wide range of biologically active compounds, acting as broad-utility solid-phase extraction agents, remains an important and necessary task. Selective sorbents can be easily used for highly selective and reliable extraction of specific components present in complex matrices. Herein, state-of-the-art approaches for selective isolation, preconcentration, and separation of biologically active compounds from a range of matrices are discussed. Primary focus is given to novel extraction methods for some biologically active compounds including cyclic polyols, flavonoids, and oligosaccharides from plants. In addition, application of silica-, carbon-, and polymer-based solid-phase extraction adsorbents and membrane extraction for selective separation of these compounds is discussed. Potential separation process interactions are recommended; their understanding is of utmost importance for the creation of optimal conditions to extract biologically active compounds including those with estrogenic properties.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Free radicals can cause damage in the human body when they interact with important cellular components such as DNA, or the cell membrane, making the body susceptible to many diseases such as atherosclerosis, diabetes, rheumatism, inflammation, age-related eye disease, and cancer [1, 2]. To prevent such harmful effects of free radicals, it is recommended to maintain a healthy diet, rich in phytochemicals such as polyphenols, flavonoids, oligosaccharides, and cyclic polyols including cyclitols (inositols) [3,4,5]. Most of these compounds are widely present in various fruits, herbs, and honey.
Attempts to isolate cyclic polyols from natural sources are considered highly valuable technical aims due to their wide range of applications in pharmaceutical manufacturing [6,7,8]. Altered levels of polyols have been observed in certain neurological disorders leading to diabetic neuropathy. This type of diabetes is associated with elevated levels of sorbitol and reduced levels of myo-inositol in neuronal tissues including cerebrospinal fluid [9]. myo-Inositol is classified as a member of the vitamin B complex (vitamin B8), produced from glucose-6-phosphate in humans by a highly conserved process known as the Loewus pathway [10]. Several studies have shown a wide range of biological activities of different cyclitols, including myo-inositol, d-chiro-inositol, and d-pinitol. myo-Inositol treatment has been shown to prevent biochemical changes triggered by kainate-induced status epilepticus [11]. Qureshi and Al-Bedah reported that patients suffering from clinical depression, bulimia, epilepsy, panic disorder, unipolar and bipolar depression, and obsessive–compulsive disorder taking high-dose myo-inositol supplement showed promising results [12]. Among other important properties, myo-inositol possesses insulin-mimetic properties and is efficient in lowering postprandial blood glucose [13]. Moreover, myo-inositol and d-chiro-inositol improve insulin resistance in obese women and women with gestational diabetes [14], in addition to the activities of myo-inositol and scyllo-inositol [15]. A 3-O-methyl-d-chiro-inositol called d-pinitol also has substantial pharmacological importance [13]; it has been widely used in treatment of hypertension, rheumatism, and cardiovascular diseases, as an anticonvulsant, and for acquired immunodeficiency syndrome (AIDS) and neurological disorders [16,17,18]. Therefore, inositols as pharmaceutical ingredients are in great demand worldwide.
Phytoestrogens are a class of naturally occurring plant nutrients that have the ability to cause estrogen-like effects on the body (sexual hormones) due to their structural similarity to estradiol (17-β-estradiol), the primary female sex hormone [19]. Phytoestrogens belong to a large group of substituted natural phenolic compounds known as flavonoids. Several groups of flavonoids have estrogenic properties. Among these, coumestans, prenylflavonoids, and isoflavones possess the greatest estrogenic activity [20, 21]. Several studies have reported health benefits of phytoestrogens including lowered risk of osteoporosis, carcinogenesis, atherosclerosis, brain function disorders, obesity, and menopausal symptoms [22,23,24]. Moreover, phytoestrogens play an important role in plant defense systems, mainly against fungi [25], and control female fertility to prevent overpopulation and fertility of animals that may eat them to reduce further attacks [26].
Other important bioactive compounds are oligosaccharides. Lopez et al. [27] reported that oligosaccharides improve metabolic absorption of certain minerals even when phytate is present.
Isolation, separation, and analysis of these compounds are of great interest in several fields, such as pharmaceutical and food industries, biochemistry, medical cell biology, and biotechnology research or nutrition [11, 28,29,30,31,32,33,34]. The cost of their synthesis can be considerably higher than their isolation from plants [35, 36], which are rich sources of these compounds.
The approaches to isolate and concentrate these compounds are determined by the chemical nature of the matrix, the properties of the individual component or the sum of their parts, and the method of analysis. Sample preconcentration, sample cleaning, and separation of cyclic polyols from other chemical constituents are complicated due to their high polarity. The hydrophobicity value (expressed as log P) of myo-inositol is −2.11 (Table 1). Moreover, naturally occurring flavonoids in plant tissues can also play an interesting role, making it important to consider their presence in matrices. The hydrophobicity values of different classes of flavonoids are higher than for cyclic polyols and vary in the range from about 0 to 4 (Table 1). Prior to chromatographic analysis, different solvents have been used for extraction of cyclic polyols from plants, including water [37], ethyl acetate and methanol [38], and ethyl acetate and ethanol [39].
Nowadays, nanotechnology has growing importance in the field of separation science. Several manufacturers now offer nanomaterial composites with different geometric structures coated on silica gel for use as SPE sorbents for preconcentration from and cleanup of various matrices. Supercritical fluid extraction (SFE) followed by selective concentration on modified SPE cartridges allows commercial SFE units to reach high concentration coefficients while conserving the samples.
This review is focused on introduction of extraction methods and new packing materials for SPE cartridges for use on laboratory scale for isolation and preconcentration of some bioactive compounds from plants and biological matrices. Current examples of adsorbents for isolation and preconcentration of components based on chemical interactions rely on nonselective H-bonding interactions between target analytes and surface groups. To date, some work has been done on nonporous graphitized carbon [40], silica modified with nonpolar groups [41], followed by chromatographic methods of analysis [40, 42]. These methods are too slow, typically requiring time of hours, with low selectivity that makes them useless in real applications. The aim of this review is to summarize and discuss available data on the most relevant methods for isolation, purification, preconcentration, and separation of some active compounds from plants and other biological matrices such as urine samples, sesame oil, and honey from various floral sources. These methods include conventional extraction methods, modern extraction methods, solid-phase extraction using carbon-, polymer-, and silica-based solid-phase extraction cartridges, and membrane extraction.
Extraction of Cyclic Polyols and Flavonoids
Extraction of bioactive compounds from plant materials has been intensively reviewed [43, 44]. Present interest is due to increased awareness of preventative healthcare, which could be promoted through consumption of plant material extracts. The different techniques employed for extraction of polyols and flavonoids can be classified into conventional and modern extraction methods. Conventional extraction methods include maceration and Soxhlet extraction, while the modern methods include pressurized liquid extraction (PLE), microwave-assisted extraction (MAE), ultrasound-assisted extraction (UAE), and supercritical fluid extraction (SFE). Extraction of solute using conventional extraction methods depends on the solubility of the solute from the plant in the solvent. A large quantity of solvent is often required to extract the desired solute, and/or use of elevated temperature, mechanical stirring, or shaking. On the other hand, modern extraction techniques consume less solvent and energy, with shorter extraction time, while preventing pollution.
The importance of polyols and flavonoids due to their pharmaceutical properties has attracted attention from many researchers. Isolation of these compounds from natural sources such as plants using different extraction methods has been performed to obtain the maximum possible concentrations.
Maceration is a conventional method for extraction of bioactive compounds from plant material, involving soaking of plant material in a specific solvent for a period of time, usually from a couple of hours up to several days. Ethanol and methanol are common solvents used for extraction of polyols and flavonoids by this technique, while water is often used to extract highly polar compounds such as polyols [45]. Ethanol is often preferred, probably due to environmental concerns. Cheng et al. [46] reported extraction of eight flavonoids from Gynostemma pentaphyllum using ethanol as solvent, while Sharma et al. [47] employed this method for isolation of the polyol d-pinitol from Argyrolobium roseum plant.
Soxhlet extraction is another conventional technique frequently used to isolate polyols and flavonoids from plant materials. Commonly, ethanol, methanol, and acetonitrile are used as solvent. This process involves heating a solvent to boiling, then returning the condensed vapors to the original flask. The main disadvantages of Soxhlet extraction are the time and solvent consumption, as at least 1 h and up to 72 h can be required for extraction. Various flavonoids have been extracted from leaves and flowers of Cassia angustifolia by Soxhlet extraction using ethanol in 5 h [48]. Also, some phytochemical compounds including myo-inositol and flavonoids were identified using gas chromatography by applying the same extraction technique to leaves of Tephrosia purpurea [49].
Pressurized fluid extraction is a green technique for plant material sample preparation prior to chromatographic analysis, utilizing conventional solvents at controlled temperature (50–200 °C) and pressure (10 and 1500–2000 psi). In addition, it uses less solvent (15–45 mL), requires shorter extraction time (15–25 min), and can be automated. Therefore, this technique has been widely applied in the fields of environmental, food, and pharmaceutical research. It is also known as accelerated solvent extraction (ASE) or enhanced solvent extraction (ESE). The efficacy of PLE for extraction of inositols has been studied and compared with other extraction methods. Higher inositol concentration (5.7 mg g−1) was obtained from pine (Pinus pinea L.) nuts using accelerated solvent extraction (conditions: 50 °C, 18 min, three cycles of 1.5 mL water each, 10 MPa) compared with solid–liquid extraction (3.7 mg g−1) (conditions: room temperature, 2 h, two cycles of 5 mL water each) [50]. PLE is also an effective technique for extraction of flavonoids from plant material. Bergeron et al. [51] reported extraction of different flavonoids from Scutellaria lateriflora using PLE at pressure of 10 MPa and various temperatures (85, 100, 150, 170, and 190 °C) with extraction time of 30 min in three cycles of 10 min.
Microwave-assisted extraction utilizes microwave energy to facilitate diffusion of analytes from the plant sample into the solvent. Water in plant material is responsible for absorbing the microwave energy, leading to internal superheating and cell structure perturbation, thus facilitating diffusion of biologically active compounds from the plant matrix. MAE has been applied for determination of isoflavonoids in Radix astragali [52]. The results showed that MAE achieved the highest extraction efficiency compared with other extraction methods such as Soxhlet, reflux, and ultrasonic extraction. The application of microwave extraction for isolation of flavonoids has been thoroughly reviewed by Routray and Orsat [53]. Regarding extraction of polyols, Ruiz-Aceituno et al. [54] compared the efficiency of microwave-assisted extraction and pressurized liquid extraction for extraction of inositols from artichoke external bracts using water as solvent, reporting that the final optimized MAE method at 60 °C for 3 min with 0.3 g of sample allowed extraction of slightly higher concentrations of inositol than PLE at 75 °C for 26.7 min (11.6 mg g−1 dry sample versus 7.6 mg g−1 dry sample) [54].
Ultrasound-assisted extraction is another environmentally friendly technique for extraction of bioactive compounds such as polyols and flavonoids from plants. It offers the same advantages as PLE and MAE in terms of reduced time and solvent use and lower energy consumption. This process involves use of ultrasound at frequencies ranging from 20 to 100 MHz to create cavitation bubbles in the solvent that disrupt plant cell walls and increase the surface contact between solvents and samples and the permeability of cell walls. UAE has been employed for extraction of bioactive compounds, such as polyols, flavonoids, and others. Žlabur et al. [55] investigated the influence of conventional and ultrasound-assisted extraction (frequency, time, and temperature) on the content of bioactive compounds including flavonoids obtained from lemon balm and peppermint leaves [55]. Meanwhile, Tetik and Yüksel [56] used UAE for extraction of d-pinitol from carob bods and studied the influence of different parameters such as temperature, ultrasonic power, dilution rate (material:water ratio), and time. Those authors reported that the highest concentration of d-pinitol was 11.98 g L−1, obtained using temperature of 50 °C, ultrasonic power of 207 W, dilution rate of 1:4, and extraction time of 120 min [56].
Supercritical fluid extraction (SFE) is one of the leading approaches for extraction of organic compounds from solid and in some cases semisolid matrices [57, 58]. The supercritical state is achieved when the temperature and pressure of a fluid are raised above the critical point, exhibiting unique properties in terms of compressibility, density, and viscosity that differ from those of gases or liquids in normal state. Cháfer and Berna [59] applied SFE for isolation of d-pinitol from carob pods at pressure of 20 or 30 MPa, temperature of 40 or 60 °C, and CO2 flow rate from 0.5 to 2.5 mL min−1, using water to trap the extract. The maximum extraction yield of flavonoids of approximately 4.24 mg g−1 from Maydis stigma flowers using SFE was obtained using temperature of 50.88 °C, pressure of 41.80 MPa, cosolvent amount of 2.488 mL g−1, and extraction time of 120 min [59].
Isolation of polyols from crude extracts by partition chromatography using ion-exchange resins and thin-layer chromatography results in low yields and recoveries, as well as being very expensive [60].
The advantages offered by CO2 as a fluid include its low critical temperature/pressure, low cost, high purity, low toxicity and reactivity, and higher quality of the extract [61]. For extraction of polar compounds such as polyols, a modifier polar cosolvent such as methanol, ethanol, acetonitrile, acetone, water, ethyl ether or dichloromethane can be added to increase the solubility. Also, an increase in the pressure of the supercritical fluid increases the solvent dipole moment and thus its “polarity.” Undoubtedly, SFE is one of the promising techniques for extraction of polyols and flavonoids.
Solid-Phase and Membrane Extraction as Purification and Separation Techniques
Besides cyclic polyols and flavonoids, plant crude extracts usually contain a mixture of different compounds such as chlorophyll, carbohydrates, sugars, fats, and others. Therefore, no universal extraction procedure is suitable for extraction of just polyols or flavonoids. Different strategies including liquid–liquid partitioning, column chromatography, membrane extraction, and solid-phase extraction (SPE) are commonly used to remove unwanted components and obtain concentrated fractions of targets before analysis.
Membrane Extraction as Separation Method for Flavonoids
Membrane extraction is an obvious alternative to classical liquid–liquid extraction due to the small volumes of organic solvent and high ratio of target sample to extractant [67, 68]. The most common membrane materials are cellulose acetate [69], polyamide [70], and polysulfone [71, 72]. A novel polysulfone–Fe3O4 composite ultrafiltration membrane was prepared and used to purify flavonoids from Ginkgo biloba extract (GBE) by adjusting the external magnetic field [73]. It was found that the content of flavonoids was superior when extraction was performed in a magnetic field. Zhang et al. [74] used a molecularly imprinted membrane prepared using luteolin as template molecule and aminopropyltriethoxysilane for separation of flavonoids.
Solid-Phase Extraction (SPE)
SPE is a good choice as cleanup procedure for crude plant extracts, since it is rapid, reproducible, economical, and sensitive, clean extracts are obtained, and different cartridges with different sorbents can be used. Carbon- and polymer-based solid-phase extraction cartridges have been developed for separation and purification of some bioactive compounds including polyols, flavonoids, and oligosaccharides.
Carbon-Based Solid-Phase Extraction Cartridges
Carbon-based adsorbents are characterized by their polydisperse particle structure. However, under specific conditions, e.g., chemical activation of biomass waste sawdust using KOH, adsorbents with a desired narrow pore size distribution (D = 0.77–0.91 nm), high surface area (S BET = 2000–3100 m2 g−1), and large micropore volume (V p = 1.11–1.68 cm3 g−1) can be obtained [75]. Most carbon adsorbents [76,77,78] possess a heterogeneous pore network formed of macropores with width exceeding about 50 nm, mesopores with width from 2 to 50 nm, and micropores with width not exceeding about 2 nm.
Granular activated carbons are typical carbon-based adsorbents. These are nongraphitic and nongraphitizable carbons with highly disordered microstructure, i.e., “a non-graphitic carbon which cannot be transformed into graphitic carbon by high-temperature treatment up to 3026.85 °C under atmospheric pressure or a lower pressure” [79]. The pore network of carbon-based adsorbents and the chemical nature of the carbon surface appear to be the criteria that determine their sorption properties. Spaces between crystals form micropores where aromatic rings render the surface hydrophobic. The structure of the carbon skeleton can be considered a mixture of graphite-like crystallites forming hexagonal nets resembling a honeycomb, where each ring is formed from six carbon atoms with delocalized π-electrons. The surface chemistry of activated carbons creates oxygen-containing functionalities, the most important of which are carboxyl, hydroxyl, ether, carbonyl, lactones, and quinine [80].
Application of activated carbon (charcoal) in sample preparation of cyclic polyols and similar compounds is discussed here. Carbon-based classical adsorbents for fractionation of OH-containing organic compounds are produced from natural precursors such as coal, wood or coconut shell after treatment with different reagents such as acids and hydroxides. Activated coal has high specific surface area (S BET > 500–1000 m2 g−1). Carbon-based sorbents are used in the form of nonporous graphitized carbon for fractionation and purification of cyclic polyols by SPE [40]. Redmond and Packer [81] described the application of SPE of oligosaccharides from dilute solutions using graphitized carbon, demonstrating its potential for purification and fractionation of mixtures of sugars based on their size and/or ionic character.
The feasibility of extracting natural organic compounds (flavonoids) from plant tissue or seed oil using limited amounts of carbon-based adsorbents was described recently [82]. Multiwalled carbon nanotubes (MWCNTs) have been reported as solid-phase adsorbents to extract polyphenols from honey samples [83,84,85]. Novel magnetic carboxylated MWCNTs treated with nitric acid, hydrogen peroxide, sulfuric acid, or a mixture of potassium permanganate and sulfuric acid were shown to be powerful sorbents capable of extracting small amounts of organic compounds [82]. Typically, any surface modification of a carbon-based adsorbent may alter its surface chemical properties and absorption capacities. The strategies available to fabricate carboxyl-containing nanotubes (c-MWCNTs) for use with various analytes have recently been expanded [86]. Furthermore, magnetic Fe3O4 particles were selected for the magnetic core because of their rapid, cheap, and high-yield synthesis, as well as strong magnetic separation properties [82]. Xiao et al. [87] have developed MWCNTs@SiO2 nanoparticles for simultaneous extraction and determination of traces of flavonoids. Multi- and single-walled carbon nanotube screen-printed electrodes (MWCNTs–SPE and SWCNTs–SPE) were used as sensors for flavonoids in presence of biocatalytic laccases, i.e., multi-copper proteins that use molecular oxygen to oxidize various aromatic and nonaromatic compounds by a radical-catalyzed reaction mechanism [88].
Carbon fibers coated with different stationary phases, which can be liquid (polymer) or solid (sorbent), can be used to extract different types of analyte. The approach and details of these newly synthesized carbon fibers (E-HyCu) have not been clarified due to a pending patent application by Sugitate and Saka [89]. However, carbon fibers play the role of two adsorbents: one with hydrophobic (C18) and the other with hydrophilic (carbon/NH2) groups. The efficiency of these new carbon fibers for removal of fatty acids and quercetin (flavonoid) was almost 100%. Deka and Saikia [90] also studied the separation and purification of flavonoids using activated carbon. Meanwhile, Ribeiro et al. [91] investigated selective removal of flavonoids from citrus juice using different adsorbents including granulated activated carbon, activated diatomaceous earths, and synthetic neutral resins (Amberlite XAD-4, XAD-7, and XAD-16). Since removal of reducing sugars, pigments, and vitamin C may also occur, the eventual adsorption of these compounds was also investigated.
Nanocomposites consisting of graphene oxide and Fe3O4 nanoparticles have been used as magnetic sorbents for extraction of flavonoids from green tea, red wine, and urine samples [92]. A solid-phase extraction method for efficient analysis of flavonoids using poly(1-vinyl-3-hexylimidazolium bromide)-graphene oxide-grafted silica has also been established and described [93]. Graphene-encapsulated silica was used as adsorbent in matrix solid-phase dispersion extraction of polymethoxylated flavonoids from dried Murraya paniculata (L.) Jack leaves [94] with recoveries above 92.61%.
Polymer-Based Solid-Phase Extraction Cartridges
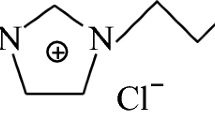
Polymer-based adsorbents are the most prominently used sorbents in SPE purification processes [95,96,97], due to their favorable stability and structural diversity, which allow modulation of their adsorption capacity and selectivity. Macroreticular polymeric resins have been widely used for adsorption, extraction, and purification of bioactive compounds from aqueous solutions and can be easily regenerated using appropriate elution solvent. Their adsorption of flavonoids can be ascribed to a synergy between hydrogen-bonding, electrostatic, π–π, and hydrophobic interactions of flavonoids with the sorbent. Synthetic neutral resins Amberlite XAD-4, XAD-7, and XAD-16 (Fig. 1) were tested for isolation of flavonoids from juice [91]. Amberlite XAD-4 and XAD-16 present similar structures, both being polystyrene-based resins, while XAD-7 is a polyacrylic-type resin. Amberlite XAD-7 has oxygen atoms in its structure and can form additional hydrogen bonds between two polar groups. Polyacrylic-type resin therefore might exhibit better absorption capacity towards polar compounds compared with Amberlite XAD-4 or XAD-16.
To address all the problems related to activated carbon, polymers with nonpolar and polar surfaces, particularly Amberlite XAD-4 based on styrene–divinylbenzene, cyano-functionalized porous polystyrene [98], biochars functionalized with polyethylimine, orthosilicate, and polyaniline [99], polyamide resins [100], aminophenyl boronic acid with uniform-macroporous poly(chloromethylstyrene-co-divinylbenzene), poly(chloromethylstyrene-co-divinylbenzene) [101], and polystyrene-divinyl sorbents with different degrees of crosslinking such as LiChrolut [102] or AMBERCHROM [103] were evaluated for solid-phase extraction of flavonoids from plant extracts.
The hydrophobic surface of Amberlite XAD-4 results in weak contact between surface groups and hydrophobic groups of flavonoids, as well as low capacity for flavonoids compared with activated carbon [104, 105]. Analogs of Amberlite XAD-4 form donor–acceptor adducts with adsorbent molecules [106, 107]. Treatment of Amberlites with solvents (methanol, acetone, or acetonitrile) slightly increases their capacity but makes the SPE process more complex. Penner et al. [108] synthesized a new class of copolymers with higher capacity towards organic chemicals compared with copolymers such as the Amberlites. An example of such material is polysterene resin with high degree of crosslinking [108]. Its enhanced specific surface area (1500 m2 g−1) and bimodal porous structure resulted in increased recovery of organic compounds compared with polystyrene-divinylbenzene sorbents such as LiChrolut, ENVI-Chrom, or AMBERCHROM with 60% degree of crosslinking. Over the last few decades, chemically modified polymers have been applied for SPE, including β-cyclodextrin cross-linked polymer [109, 110] and different acrylic copolymers [67] containing CN–, –CONH2, and –COOH groups with different degrees of substitution, as presented in Fig. 2.
This led to the introduction of smart adsorbents with a molecular fingerprint, called molecularly imprinted polymers (MIPs) in literature, offering significantly improved selective isolation [111, 112]. This approach is based on noncovalent imprinting, which involves formation of a host–guest complex via hydrogen bonds, ionic interactions, hydrophobic interactions, and metal ion interactions [113]. Figure 3 illustrates the synthesis process of MIPs, which mainly consists of three steps: (1) formation of a complex between the functional monomers and the template molecules through covalent or noncovalent interactions, (2) polymerization of the functional monomers in the presence of polymeric cross-linking reagent, and (3) removal of the template molecules from the polymer matrix to create three- dimensional cavities that are sterically and chemically complementary to the target analyte.
The characteristics of the synthesized material surface can be investigated using classical physicochemical methods such as porosimetry, characterization of pore distribution, pore volume, pore diameter, and surface area, scanning electron microscopy (SEM) or transmission electron microscopy (TEM), atomic force microscopy (AFM), solid-state nuclear magnetic resonance (NMR), and Fourier-transform infrared (FTIR) spectroscopy to determine surface functional groups. The surface properties of thus-prepared sorbents and functional groups significantly influence their selectivity towards and recovery of target analytes (Fig. 4).
Another new group of adsorbents based on MIP materials are so-called core–shell materials (Fig. 5a), whose sorption capacity and specificity can be controlled via their design, architecture, and physicochemical properties, for example, polymeric sorbents with nonporous center and porous outer layer as a mantle (Fig. 5b), in which magnetic Fe3O4 nanoparticles are localized. These materials, both polymeric and silica based, have been successfully used to purify the supernatant of complex biological samples (plant and animal tissues) [113].
Metal–organic frameworks (MOFs) are porous hybrid materials with a periodic three-dimensional network, possessing high porosity and specific surface area (Fig. 5c). Such materials are therefore characterized by high sorption capacity, which is important for sample preparation, especially in liquid–solid systems. Due to their merits, MOF materials have been widely used as candidates for molecular separation, gas storage, and catalysis [114]. The variety of applications and the ability for functionalization of the surface significantly influence the selectivity of the isolation process. Examples of such materials include polysaccharide-based sorbents such as cyclodextrins (CDs).
Cyclodextrins (CD) are cyclic oligosaccharides formed with D(+) glucopyranoside units bonded to each other through α(1 → 4) glycosidic bonds. Primary and secondary alcohol groups are located on the outer surface of the molecule. CDs have a hydrophobic inner core, while the outer surface of the cone is hydrophilic. Molecules of polar analytes form inclusion complexes with CDs through H-bonding. CD-based polymers are characterized by low specific surface area and the lack of a permeable porous network. This results in weaker interaction between the molecules of analytes and the grafted CD molecules.
Cartridges filled with HySphere GP (polymeric polydivinylbenzene phase) and HySphere SH (strong-hydrophobic modified polystyrene-divinylbenzene) have been proposed in literature [115, 116]. Although these polymers are selective towards flavonoids, their use can be time-consuming. However, they can decrease the limit of detection several fold due to the increased sample volume. Strong interactions between adsorbents and analytes result in lower limit of detection. Recovery of flavonoids using such adsorbents occurs as a result of nonspecific sorption by the matrix, formation of donor–acceptor adducts, or recovery due to an ion-exchange mechanism between surface-bound weak (or strongly basic) and weakly acidic groups of flavonoids.
The aromatic rings and carbonyl groups of Amberlites behave as Lewis bases, while flavonoids behave as Lewis acids. Therefore, recovery of flavonoids will be easiest from media where they exist in molecular form. In recent years, polystyrene-divinylbenzene-based copolymers containing polar groups such as carbonyl, boronic acid, sulfonic acid, and nitrile groups have also been developed, where recovery occurs as a result of π-interactions or formation of hydrogen bonds. Imidazole polymers, methylimidazole polymers, carboxyl-imidazole polymers, and amino-imidazole polymers have also been employed for separation of polysaccharides [117] (Fig. 6). Polymers with a layer of copper phthalocyanine on polymer sorbent POLYSORB-2 represent additional examples of applications of polymers for SPE of antioxidants [118].
Chelation affects the formation of ionic bonds, resulting in higher affinity of the functional polymer towards diol-containing compounds. Both modified and unmodified organic polymers have been used to preconcentrate different classes of organic compound, including phenolic compounds, aldehydes, ketones, carboxylic acids, esters, and ethers [97, 119]. All the discussed polymer-based materials contain hydrophobic moieties (alkyl chains and aromatic groups) as well as hydrophilic groups. The hydrophilic fragments can be divided into neutral hydrophilic groups (cyano, amide, carbonyl), ionic groups (carboxyl, amino, sulfonic, imidazole ring), and zwitterionic groups (aminophenyl boronic acid). The complexity of ion-exchange sites (hydrophilic and hydrophobic moieties) leads to a lack of selectivity, poor recovery kinetics, and the need to use organic solvents, forcing researchers to seek more effective sorbents for SPE.
Other SPE Sorbents
In addition to the solvent and the pH stability of carbon and/or polymeric adsorbents in the range of 1–14, silica materials are still in use, mainly for nonpolar or medium-polar analytes. Silica-based sorbents are modified with groups such as C1, C2, C8, C18, CH, Ph or CN. One of the disadvantages of the most popular silica-based materials is the presence of residual silanol groups. When the water–organic mixture comes into contact with the stationary phase, organic solvent molecules are adsorbed onto the bonded functional groups via hydrophobic interactions, while water can adsorb on residual silanols via hydrogen bonding [113]. Moreover, presence of residual silanols can negatively influence the separation of polar analytes, especially basic compounds or biopolymers [113, 120, 121]. Additionally, to obtain more efficient recovery and a totally polar sorbent, the trends are to minimize the number of residual groups on the original silica. Furthermore, a trifunctional silane has been used for bonding n-alkyl chains with end-capping performed via trimethylsilane after bonding [122].
Stationary phases for adsorption include unmodified sorbents such as pure/bare silica, alumina, magnesium silicate, and diatomaceous earth. Their hydrogen-binding sites are mainly destroyed by water, which results in reduced retention of compounds of interest and poor reproducibility. For this reason, the surface of silica is functionalized by attaching polar groups such as cyano, amine, amide, and diol, resulting in a normal-phase sorbent that can extract a polar analyte from a polar medium via hydrophilic interactions. Such materials are mainly applied in clinical fields for purification of non-polar extracts such as hexane [123].
Ion-exchange sorbents are characterized by ionic interactions, as in the case of negatively and positively charged analytes and biological fluids. Optimal conditions, including pH values, should be ensured. For this reason, depending on the nature of the analyte, use of new weak or strong cation- or anion-exchange stationary phases is recommended. Some of these contain specific active centers for selective isolation of analytes such as cations or anions. It is possible to control the polarity of both the stationary phase (surface shielding) and mobile phase (pH change) (Fig. 7).
The relationship between a target compound and its separation on the solid phase is mediated by hydrophobic interactions such as van der Waals forces as well as hydrophilic interactions such as dipole–dipole, induced dipole–dipole, hydrogen bonding, and π-interactions. Additionally, between the charged groups on the analyte of interest and the sorbent surface, electrostatic attractions as well as molecular recognition mechanisms are relevant. These types of interaction have been characterized for reversed-phase, normal-phase, ion-exchange, immunoaffinity, and molecularly imprinted polymers.
Conclusions
This review has presented an overview of extraction techniques and separation methods for isolation of various biologically active compounds including cyclic polyols, flavonoids, and oligosaccharides from plant sources, as well as the advantages and disadvantages of each technique, and provided information about SPE as a method for separation and sample preparation before analysis. In addition, some commercial and synthetic SPE sorbents and their application for separation of selected components were presented. Conventional extraction methods were probably the first to be used for extraction of bioactive compounds present in plant materials using appropriate solvents. Compared with those methods, modern extraction techniques consume less solvent, hazardous chemicals, and energy, save time, and prevent pollution. SPE represents the best alternative compared with other purification methods for isolation of chemical constituents of plants. The long contact time during sorption of target analytes on activated carbons, as well as their universality towards different classes of organic compound, make them preferable for purification of extracts compared with other SPE sorbent materials. Polymers with a wide variety of functional groups, surface area, and porosity have become clear alternatives to activated carbons. The sorption properties of polymer-based adsorbents depend on their structure, the type of chemical bond between their chains, as well as their elementary structural units, molecular mass, and composition.
Synthesis of new materials to achieve optimal conditions for selective isolation of cyclic polyols and flavonoids still requires further research and development. Such materials could represent promising alternatives for selective isolation and separation of biologically active compounds from plants, including those with estrogenic properties.
Abbreviations
- CD:
-
Cyclodextrin
- MAE:
-
Microwave-assisted extraction
- MIP:
-
Molecularly imprinted polymers
- MOF:
-
Metal–organic frameworks
- MWCNTs:
-
Multiwalled carbon nanotubes
- c-MWCNTs:
-
Carboxyl-containing multiwalled carbon nanotubes
- PLE:
-
Pressurized liquid extraction
- SFE:
-
Supercritical fluid extraction
- SPE:
-
Solid-phase extraction
References
Ambigaipalan P, de Camargo AC, Shahidi F (2017) Identification of phenolic antioxidants and bioactives of pomegranate seeds following juice extraction using HPLC-DAD-ESI–MSn. Food Chem 221:1883–1894
Ahmad SR, Gokulakrishnan P, Giriprasad R, Yatoo MA (2015) Fruit-based natural antioxidants in meat and meat products: a review. Crit Rev Food Sci Nutr 55(11):1503–1513
Parazzini F (2014) Resveratrol, inositol, vitamin D and K in the prevention of cardiovascular and osteoporotic risk: a novel approach in peri- and postmenopause. Minerva Ginecol 66:513–518
Celentano C, Matarrelli B, Mattei PA, Pavone G, Vitacolonna E, Liberati M (2016) Myo-inositol supplementation to prevent gestational diabetes mellitus. Curr Diab Rep 16:30
Sun Y, Jiang L, Wei D (2013) Partial characterization, in vitro antioxidant and antiproliferative activities of patatin purified from potato fruit juice. Food Funct 4:1502
van Wyk BE, Albrecht C (2008) A review of the taxonomy, ethnobotany, chemistry and pharmacology of Sutherlandia frutescens (Fabaceae). J Ethnopharmacol 119:620–629
Sharma S, Chattopadhyay SK, Singh M, Bawankule DU, Kumar S (2014) Novel chemical constituents with anti-inflammatory activity from the leaves of Sesbania aculeata. Phytochemistry 100:132–140
Rafińska K, Pomastowski P, Wrona O, Górecki R, Buszewski B (2017) Medicago sativa as a source of secondary metabolites for agriculture and pharmaceutical industry. Phytochem Lett 20:520–539
Shetty HU, Holloway HW, Rapoport SI (1995) Capillary gas chromatography combined with ion trap detection for quantitative profiling of polyols in cerebrospinal fluid and plasma. Anal Biochem 224:279–285
Pazourek J (2014) Fast separation and determination of free myo-inositol by hydrophilic liquid chromatography. Carbohydr Res 391:55–60
Solomonia R, Mikautadze E, Nozadze M, Kuchiashvili N, Lepsveridze E, Kiguradze T (2010) Myo-inositol treatment prevents biochemical changes triggered by kainate-induced status epilepticus. Neurosci Lett 468:277–281
Qureshi NA, Al-Bedah AM (2013) Mood disorders and complementary and alternative medicine: a literature review. Neuropsychiatr Dis Treat 9:639–658
Koletzko B, Bhutta ZA, Cai W, Cruchet S, El Guindi M, Fuchs GJ, Elizabeth A, Goddard EA, van Goudoever JB, Quak SH, Kulkarni B, Makrides M, Ribeiro H, Walker A (2013) Compositional requirements of follow-up formula for use in infancy: recommendations of an international expert group coordinated by the early nutrition academy. Ann Nutr Metab 62:44–54
Ferrari F, Facchinetti F, Ontiveros AE, Roberts RP, Saade MM, Blackwell SC, Sibai BM, Refuerzo JS, Longo M (2016) The effect of combined inositol supplementation on maternal metabolic profile in pregnancies complicated by metabolic syndrome and obesity. Am J Obstet Gynecol 215:503e1–503e8
Nozadze M, Mikautadze E, Lepsveridze E, Mikeladze E, Kuchiashvili N, Kiguradze T, Kikvidze M, Solomonia R (2011) Anticonvulsant activities of myo-inositol and scyllo-inositol on pentylenetetrazol induced seizures. Seizure 20:173–176
Chauhan PS, Gupta KK, Bani S (2011) The immunosuppressive effects of Agyrolobium roseum and pinitol in experimental animals. Int Immunopharmacol 11:286–291
Davis A, Christiansen M, Horowitz JF, Klein S, Hellerstein MK, Ostlund RE Jr (2000) Effect of pinitol treatment on insulin action in subjects with insulin resistance. Diabetes Care 23:1000–1005
Singh RK, Pandey BL, Tripathi M, Pandey VB (2001) Anti-inflammatory effect of (+)-pinitol. Fitoterapia 72:168–170
Ričanyová J, Gadzała-Kopciuch R, Reiffová K, Buszewski B (2009) Estrogens and their analytics by hyphenated separation techniques. Crit Rev Anal Chem 39:13–31
Bingham SA, Atkinson C, Liggins J, Bluck L, Coward A (1998) Phyto-oestrogens: where are we now? Br J Nutr 79:393–406
Murkies AL, Wilcox G, Davis SR (1998) Phytoestrogens. J Clin Endocrinol Metab 83(2):297–303
Messina MJ, Persky V, Setchell KD, Barnes S (1994) Soy intake and cancer risk: a review of the in vitro and in vivo data. Nutr Cancer 21:113–131
Cooke GM (2006) A review of the animal models used to investigate the health benefits of soy isoflavones. J AOAC Int 89:1215–1227
Rietjens IMCM, Louisse J, Beekmann K (2017) The potential health effects of dietary phytoestrogens. Br J Pharmacol 174:1263–1280
Blount JW, Dixon RA, Paiva NL (1992) Stress responses in alfalfa (Medicago sativa L.) XVI. Antifungal activity of medicarpin and its biosynthetic precursors; implications for the genetic manipulation of stress metabolites. Physiol Mol Plant Pathol 41:333–349
Hughes CL Jr (1988) Phytochemical mimicry of reproductive hormones and modulation of herbivore fertility by phytoestrogens. Environ Health Perspect 78:171–174
Lopez HW, Coudray C, Ballanger I, Younes H, Demigne C, Remesy C (1998) Intestinal fermentation lessens the inhibitory effects of phytic acid on mineral utilization in rats. J Nutr 128:1192–1198
Baumgartner S, Genner-Ritzmann R, Haas J, Amadb R, Neukom H (1986) Isolation and identification of cyclitols in carob pods (Ceratonia siliqua L.). J Agric Food Chem 34:827–829
Rebecca OPS, Boyce AN, Somasundram C (2012) Isolation and identification of myo-inositol crystals from dragon fruit (Hylocereus polyrhizus). Molecules 17:4583–4594
Giordano D, Corrado F, Santamaria A, Quattrone S, Pintaudi B, Di Benedetto A, D’Anna R (2011) Effects of myo-inositol supplementation in postmenopausal women with metabolic syndrome. Menopause 18:102–104
Browne CA, Forbes TP, Sisco E (2016) Detection and identification of sugar alcohol sweeteners by ion mobility spectrometry. Anal Methods 8:5611–5618
Schimpf KJ, Meek CC, Leff RD, Phelps DL, Schmitz DJ, Cordle CT (2015) Quantification of myo-inositol, 1,5-anhydro-d-sorbitol, and d-chiro-inositol using high-performance liquid chromatography with electrochemical detection in very small volume clinical samples. Biomed Chromatogr 29:1629–1636
Ruiz-Aceituno L, Rodríguez-Sánchez S, Ruiz-Matute AI, Ramos L, Soria AC, Sanz ML (2013) Optimisation of a biotechnological procedure for selective fractionation of bioactive inositols in edible legume extracts. J Sci Food Agric 93:2797–2803
Cunha AG, da Silva AAT, Godoy MG, Almeida RV, Simas ABC, Freire DMG (2013) Experimental design of the kinetic resolution of a key precursor of high-value bioactive myo-inositols by an immobilized lipase. J Chem Technol Biotechnol 88:205–211
Hudlicky T, Price JD, Rulin F, Tsunoda T (1990) Efficient and enantiodivergent synthesis of (+)- and (−)-pinitol. J Am Chem Soc 112:9439–9440
Duchek J, Adams DR, Hudlicky T (2011) Chemoenzymatic synthesis of inositols, conduritols, and cyclitol analogues. Chem Rev 111:4223–4258
Rodríguez-Sánchez S, Ruiz-Matute AI, Alañón ME, Pérez-Coello MS, de Julio-Torres LF, Morales R, Martínez-Castro I (2010) Analysis of cyclitols in different Quercus species by gas chromatography-mass spectrometry. J Sci Food Agric 90:1735–1738
Sukantha TA, Shubashini K (2015) Isolation and characterization of secondary metabolites from Pithecellobium dulce Benth fruit peel. Int J Pharm Pharm Sci 7:199–203
Duquesnoy E, Castola V, Casanova J (2008) Identification and quantitative determination of carbohydrates in ethanolic extracts of two conifers using 13C NMR spectroscopy. Carbohydr Res 343:893–902
Grys W, Kaluzna-Czaplinska J, Rynkowski J (2013) Gas chromatography-electron capture detection method for determination of polyols in the urine. Curr Org Chem 17:2359–2364
Miyagi M, Yokoyama H, Hibi T (2007) Sugar microanalysis by HPLC with benzoylation: improvement via introduction of a C-8 cartridge and a high efficiency ODS column. J Chromatogr B 854:286–290
Carvalho A, Pio C, Santos C (2003) Water-soluble hydroxylated organic compounds in German and Finnish aerosols. Atmos Environ 37:1775–1783
Al-Suod A, Ligor M, Rafinska K, Górecki R, Buszewski B (2017) A window on cyclitols: characterization and analytics of inositols. Phytochem Lett 20:507–519
Stalikas CD (2007) Extraction, separation, and detection methods for phenolic acids and flavonoids. J Sep Sci 30:3268–3295
Rodríguez-Sánchez S, Ruiz-Aceituno L, Sanz ML, Soria AC (2013) New methodologies for the extraction and fractionation of bioactive carbohydrates from mulberry (Morus alba) leaves. J Agric Food Chem 61:4539–4545
Cheng TC, Lu JF, Wang JS, Lin LJ, Kuo HI, Chen BH (2011) Antiproliferation effect and apoptosis mechanism of prostate cancer cell PC-3 by flavonoids and saponins prepared from Gynostemma pentaphyllum. J Agric Food Chem 59:11319–11329
Sharma N, Verma KM, Gupta DK, Satti NK, Khajuria RK (2016) Isolation and quantification of pinitol in Argyrolobium roseum plant, by 1H-NMR. J Saudi Chem Soc 20:81–87
Laghari AQ, Memon S, Nelofar A, Laghari AH (2011) Extraction, identification and antioxidative properties of the flavonoid-rich fractions from leaves and flowers of Cassia angustifolia. Am J Anal Chem 2:871–878
Suresh C, Senthilkumar S, Vijayakumari K (2012) GC–MS analysis of Tephrosia Purpurea Pers leaf extract-traditional valuable plant. Int J Pharm Life Sci 2:86–91
Ruiz-Aceituno L, Rodríguez-Sánchez S, Sanz J, Sanz ML, Ramos L (2014) Optimization of pressurized liquid extraction of inositols from pine nuts (Pinus pinea L.). Food Chem 153:450–456
Bergeron C, Gafner S, Clausen E, Carrier DJ (2005) Comparison of the chemical composition of extracts from Scutellaria lateriflora using accelerated solvent extraction and supercritical fluid extraction versus standard hot water or 70% ethanol extraction. J Agric Food Chem 53:3076–3080
Song JZ, Mo SF, Yip YK, Qiao CF, Han QB, Xu HX (2007) Development of microwave assisted extraction for the simultaneous determination of isoflavonoids and saponins in radix astragali by high performance liquid chromatography. J Sep Sci 30(6):819–824
Routray W, Orsat V (2005) Microwave-assisted extraction of flavonoids: a review. Food Bioprocess Technol 5:409–424
Ruiz-Aceituno L, García-Sarrió MJ, Alonso-Rodriguez B, Ramos L, Sanz ML (2016) Extraction of bioactive carbohydrates from artichoke (Cynara scolymus L.) external bracts using microwave assisted extraction and pressurized liquid extraction. Food Chem 196:1156–1162
Žlabur JŠ, Voća S, Dobričević N, Pliestić S, Galić A, Boričević A, Borić N (2016) Ultrasound-assisted extraction of bioactive compounds from lemon balm and peppermint leaves. Int Agrophys 16:1–10
Tetik N, Yüksel E (2014) Ultrasound-assisted extraction of d-pinitol from carob pods using response surface methodology. Ultrason Sonochem 21:860–865
Buszewski B, Szumski M, Kowalski P (1998) Odbieralnik analitów w ekstrakcji, zwłaszcza w stanie nadkrytycznym. Patent PL 188141 B1
Ligor M, Szumski M, Buszewski B (2000) Isolation and determination of flavor and fragrance components from natural products by SPME–GC and SFE–GC methods. Int Lab 30:22–25
Cháfer A, Berna A (2014) Study of kinetics of the d-pinitol extraction from carob pods using supercritical CO2. J Supercrit Fluids 94:212–215
Liu J, Lin S, Wang Z, Wang C, Wang E, Zhang Y, Liu J (2011) Supercritical fluid extraction of flavonoids from Maydis stigma and its nitrite-scavenging ability. Food Bioprod Process 89:333–339
Imam MU, Ismail M, Ooi DJ, Azmi NH, Sarega N, Chan KW, Bhanger MI (2015) Are bioactive-rich fractions functionally richer? Crit Rev Biotechnol 36:1–9
Oh M-M, Rajashekar CB (2009) Antioxidant content of edible sprouts: effects of environmental shocks. J Sci Food Agric 89:2221–2227
Hanif MA, Al-Maskari AY, Al-Sabahi JN, Al-Hdhrami I, Khan MM, Al-Azkawi A, Hussain AI (2015) Chemical characterisation of bioactive compounds in Medicago sativa growing in the desert of Oman. Nat Prod Res 29:2332–2335
Gatouillat G, Magid AA, Bertin E, El btaouri H, Morjani H, Lavaud C, Madoulet C (2015) Medicarpin and millepurpan, two flavonoids isolated from Medicago sativa, induce apoptosis and overcome multidrug resistance in leukemia P388 cells. Phytomedicine 22:1186–1194
Marles MAS, Ray H, Gruber MY (2003) New perspectives on proanthocyanidin biochemistry and molecular regulation. Phytochemistry 64:367–383
Aufrère J, Theodoridou K, Mueller-Harvey I, Yu P, Andueza D (2014) Ruminal dry matter and nitrogen degradation in relation to condensed tannin and protein molecular structures in sainfoin (Onobrychis viciifolia) and lucerne (Medicago sativa). J Agric Sci 152:333–345
Tasselli F, Donato L, Drioli E (2008) Evaluation of molecularly imprinted membranes based on different acrylic copolymers. J Membr Sci 320:167–172
Kobayashi T, Ying Wang H, Fujii N (1998) Molecular imprint membranes of polyacrylonitrile copolymers with different acrylic acid segments. Anal Chim Acta 365:81–88
Yoshikawa M, Ooi T, Izumi J-I (1999) Alternative molecularly imprinted membranes from a derivative of natural polymer, cellulose acetate. J Appl Polym Sci 72:493–499
Screenivasulu Reddy P, Kobayashi T, Abe M, Fujii N (2002) Molecular imprinted Nylon-6 as a recognition material of amino acids. Eur Polym J 38:521–529
Yoshikawa M, Izumi J, Ooi T, Kitao T, Guiver MD, Robertson PG (1998) Carboxylated polysulfone membranes having a chiral recognition site induced by an alternative molecular imprinting technique. Polym Bull 40:517–524
Cassano A, Conidi C, Ruby-Figueroa R (2014) Recovery of flavonoids from orange press liquor by an integrated membrane process. Membranes (Basel) 4:509–524
He Y-H, Zhou L, Gao B, Feng F, Fan X-J, Pan C-P, Liu Q-J, Chu X-G (2013) The characterization of polysulfone–Fe3O4 composite ultrafiltration membrane and its application. Desalin Water Treat 51:5494–5500
Zhang Y, Shan X, Gao X (2011) Development of a molecularly imprinted membrane for selective separation of flavonoids. Sep Purif Technol 76:337–344
Cheng F, Liang J, Zhao J, Tao Z, Chen J (2008) Biomass waste-derived microporous carbons with controlled texture and enhanced hydrogen uptake. Chem Mater 20:1889–1895
Ravi S, Vadukumpully S (2016) Sustainable carbon nanomaterials: recent advances and its applications in energy and environmental remediation. J Environ Chem Eng 4:835–856
Jahandar Lashaki M, Atkinson JD, Hashisho Z, Phillips JH, Anderson JE, Nichols M (2016) The role of beaded activated carbon’s pore size distribution on heel formation during cyclic adsorption/desorption of organic vapors. J Hazard Mater 315:42–51
Masson S, Gineys M, Delpeux-Ouldriane S, Reinert L, Guittonneau S, Beguin F, Duclaux L (2016) Single, binary, and mixture adsorption of nine organic contaminants onto a microporous and a microporous/mesoporous activated carbon cloth. Microporous Mesoporous Mater 234:24–34
Ebbesen TW, Takada T (1995) Topological and SP3 defect structures in nanotubes. Carbon (New York) 33:973–978
Buszewske B, Michel M (2008) Porous graphitized carbon as a stationary phase for separation technology. In: Terzyk AP, Gauden PA, Kowalczyk P (eds) Carbon materials: theory and practice. Research Signpost, Kerala
Redmond JW, Packer NH (1999) The use of solid-phase extraction with graphitised carbon for the fractionation and purification of sugars. Carbohydr Res 319:74–79
Wu R, Ma F, Zhang L, Zhang L, Li P, Li G, Zhang Q, Zhang W, Wang X (2016) Simultaneous determination of phenolic compounds in sesame oil using LC–MS/MS combined with magnetic carboxylated multi-walled carbon nanotubes. Food Chem 204:334–342
Badjah Hadj Ahmed AY, Wabaidur SM, Siddiqui MR, Alothman ZA, Obeid MS, Khan MR, AL-Tamrah SA (2016) Simultaneous determination of twenty-five polyphenols in multifloral and cactus honeys using solid-phase extraction and high-performance liquid chromatography with photodiode array detection. Eur Food Res Technol 242:943–952
Wabaidur SM, Ahmed YBH, Alothman ZA, Obbed MS, AL-Harbi NM, AL-Turki TM (2015) Ultra high performance liquid chromatography with mass spectrometry method for the simultaneous determination of phenolic constituents in honey from various floral sources using multiwalled carbon nanotubes as extraction sorbents. J Sep Sci 38:2597–2606
Badjah Hadj Ahmed AY, Obbed MS, Wabaidur SM, AlOthman ZA, Al-Shaalan NH (2014) High-performance liquid chromatography analysis of phenolic acid, flavonoid, and phenol contents in various natural Yemeni honeys using multi-walled carbon nanotubes as a solid-phase extraction adsorbent. J Agric Food Chem 62:5443–5450
Han JT, Jeong HJ, Lee G-W (2008) Buckypaper from thin multiwalled carbon nanotubes. In: Razeghi M, Pribat D, Lee YH (eds) SPIE. San Diego, CA, USA
Xiao D, Yuan D, He H, Pham-Huy C, Dai H, Wang C, Zhang C (2014) Mixed hemimicelle solid-phase extraction based on magnetic carbon nanotubes and ionic liquids for the determination of flavonoids. Carbon 72:274–286
Di Fusco M, Tortolini C, Deriu D, Mazzei F (2010) Laccase-based biosensor for the determination of polyphenol index in wine. Talanta 81:235–240
Sugitate K, Saka M (2015) Decrease in the matrix enhancement effect on pesticides analysis with GC–MS using new types of solid-phase extraction column. J Pestic Sci 40:87–91
Deka H, Saikia MD (2015) Structural and thermodynamic factors on adsorptive interaction of certain flavonoids onto polymeric resins and activated carbon. Colloids Surf A Physicochem Eng Asp 469:51–59
Ribeiro M, Silveira D, Ferreira-Dias S (2002) Selective adsorption of limonin and naringin from orange juice to natural and synthetic adsorbents. Eur Food Res Technol 215:462–471
Wu J, Xiao D, Zhao H et al (2015) A nanocomposite consisting of graphene oxide and Fe3O4 magnetic nanoparticles for the extraction of flavonoids from tea, wine and urine samples. Microchim Acta 182:2299–2306
Hou X, Liu S, Zhou P, Li J, Liu X, Wang L, Guo Y (2016) Polymeric ionic liquid modified graphene oxide-grafted silica for solid-phase extraction to analyze the excretion-dynamics of flavonoids in urine by Box-Behnken statistical design. J Chromatogr A 1456:10–18
Sun T, Li X, Yang J, Li L, Jin Y, Shi X (2015) Graphene-encapsulated silica as matrix solid-phase dispersion extraction sorbents for the analysis of poly-methoxylated flavonoids in the leaves of Murraya panaculata (L.) Jack. J Sep Sci 38:2132–2139
Fumes BH, Silva MR, Andrade FN, Nazario CED, Lanças FM (2015) Recent advances and future trends in new materials for sample preparation. TrAC Trends Anal Chem 71:9–25
Tian M, Qiao J, Row KH (2013) Facile preparation of an ionic liquid composite mesoporous polymer as a solid phase extraction adsorbent for the separation and purification of flavonoids from Chamaecyparis obtusa. Anal Lett 46:1331–1341
Tian M (2015) Current extraction methods and purification materials for bioactive compounds from natural plants. Asian J Chem 27:1961–1966
Nam J-B, Ryu J-H, Kim J-W, Chang I-S, Suh K-D (2005) Stabilization of resveratrol immobilized in monodisperse cyano-functionalized porous polymeric microspheres. Polymer (Guildf) 46:8956–8963
Yadav V, Shrivastava P, Deshmukh Y, Shanker K, Khare P (2016) Evaluation of solid phase extraction efficiency of functionalized biochar for polyphenols from Punica granatum. Asia Pac J Chem Eng 11:200–208
Xiao R, Li-mei Z, Xing-xing G, Yan LY, Zhang H, Zhu ZY, Wang Q, Jiang DA (2013) Separation and purification of flavonoid from Taxus remainder extracts free of taxoids using polystyrene and polyamide resin. J Sep Sci 36:1925–1934
Çetinkaya O, Duru ME, Çiçek H (2012) Synthese and characterization of boronic acid functionalized macroporous uniform poly(4-chloromethylstyrene-co-divinylbenzene) particles and its use in the isolation of antioxidant compounds from plant extracts. J Chromatogr B 909:51–60
Maruška A, Ragažinskienė O, Vyšniauskas O, Kaškonienė V, Bartkuvienė V, Kornyšova O, Briedis V, Ramanauskienė K (2014) Flavonoids of willow herb (Chamerion angustifolium (L.) Holub) and their radical scavenging activity during vegetation. Adv Med Sci 59:136–141
Zhang J, Chu C-J, Li X-L, Yao S, Yan B, Ren H-L, Xu N-Y, Liang Z-T, Zhao Z-Z (2014) Isolation and identification of antioxidant compounds in Vaccinium bracteatum Thunb. by UHPLC-Q-TOF LC/MS and their kidney damage protection. J Funct Foods 11:62–70
Valderrama C, Gamisans X, Heras FX, de las Heras FX, Cortina JL, Farràn A (2007) Kinetics of polycyclic aromatic hydrocarbons removal using hyper-cross-linked polymeric sorbents Macronet Hypersol MN200. React Funct Polym 67:1515–1529
Tai MH, Saha B, Streat M (1999) Characterisation and sorption performance of a Hypersol-Macronet polymer and an activated carbon. React Funct Polym 41:149–161
Li A, Zhang Q, Zhang G, Chen J, Fei Z, Liu F (2002) Adsorption of phenolic compounds from aqueous solutions by a water-compatible hypercrosslinked polymeric adsorbent. Chemosphere 47:981–989
dos Costa TS, Rogez H, da Pena RS (2015) Adsorption capacity of phenolic compounds onto cellulose and xylan. Food Sci Technol 35:314–320
Penner NA, Nesterenko PN, Rybalko MA (2001) Use of hypercrosslinked polystyrene for the determination of pyrocatechol, resorcinol, and hydroquinone by reversed-phase HPLC with dynamic on-line preconcentration. J Anal Chem 56:934–939
Zhu X, Ping W (2014) Optimization of β-cyclodextrin cross-linked polymer for monitoring of quercetin. Spectrochim Acta Part A Mol Biomol Spectrosc 132:38–43
Zhao J, Lin D-Q, Yao S-J (2012) Adsorption of rutin with a novel β-cyclodextrin polymer adsorbent: thermodynamic and kinetic study. Carbohydr Polym 90:1764–1770
Wulff G, Sarhan A (1972) The use of polymers with enzyme-analogous structures for the resolution of racemates. Angew Chem Int Ed 11:341–344
Ansari S (2017) Application of magnetic molecularly imprinted polymer as a versatile and highly selective tool in food and environmental analysis: recent developments and trends. Trends Anal Chem 90:89–106
Buszewski B, Szultka M (2012) Past, present, and future of solid phase extraction: a review. Crit Rev Anal Chem 42:198–213
Qian K, Deng Q, Fang G, Wang J, Pan M, Wang S, Pu Y (2016) Metal–organic frameworks supported surface–imprinted nanoparticles for the sensitive detection of metolcarb. Biosens Bioelectron 79:359–363
Fang J, Paetz C, Schneider B (2011) C-methylated flavanones and dihydrochalcones from Myrica gale seeds. Biochem Syst Ecol 39:68–70
Orozco-Solano MI, Ferreiro-Vera C, Priego-Capote F, Luque de Castro MD (2012) Automated method for determination of olive oil phenols and metabolites in human plasma and application in intervention studies. J Chromatogr A 1258:108–116
Zhang H, Tang B, Row KH (2015) Solid phase extraction and separation of polysaccharides from marine plant by ionic liquid-modified polymers. Asian J Chem 27:2699–2703
Faustova ZV, Slizhov YG, Gavrilenko MA, Gavrilenko NA, Terentiev RA (2015) Determination of antioxidant composition in berry juices using solid phase extraction with copper phthalocyanine. Key Eng Mater 670:213–217
Vidal L, Parshintsev J, Hartonen K, Canals A, Riekkola M-L (2012) Ionic liquid-functionalized silica for selective solid-phase extraction of organic acids, amines and aldehydes. J Chromatogr A 1226:2–10
McCalley DV (2003) Selection of suitable stationary phases and optimum conditions for their application in the separation of basic compounds by reversed-phase HPLC. J Sep Sci 26:187–200
Pichon V (2007) Selective sample treatment using molecularly imprinted polymers. J Chromatogr A 1152:41–53
Buszewski B, Jezierska M, Wełniak M, Berek D (1998) Survey and trends in the preparation of chemically bonded silica phases for liquid chromatographic analysis. J High Resolut Chromatogr 21:267–281
Buszewski B, Szultka M, Gadzała-Kopciuch R (2012) Sorbent chemistry, evolution. In: Pawliszyn J (ed) Comprehensive sampling and sample preparation, analytical techniques for scientists. Elsevier, Amsterdam
Acknowledgements
This work was partially financially supported by project Maestro-6 no. 2014/14/A/ST4/00641 (2015–2018) from the National Science Centre in Cracow (Poland) and PLANTARUM project no. BIOSTRATEG2/298205/9/NCBR/2016 from the National Centre for Research and Development in Warsaw (Poland). The authors would also like to thank Dr. Ileana-Andreea Ratiu for reading the manuscript and constructive corrections.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Additional information
Published in the topical collection 2nd ACROSS International Symposium on Advances in Separation Science (ASASS 2016) with guest editors Pavel Nesterenko and Brett Paull.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Raks, V., Al-Suod, H. & Buszewski, B. Isolation, Separation, and Preconcentration of Biologically Active Compounds from Plant Matrices by Extraction Techniques. Chromatographia 81, 189–202 (2018). https://doi.org/10.1007/s10337-017-3405-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10337-017-3405-0