Abstract
On the basis of long-term (2001–2017) and extensive data (> 1700 breeding attempts), we assess factors influencing breeding parameters in Montagu’s Harrier (Circus pygargus), a medium-sized ground-nesting semi-colonial raptor breeding in cereal fields, in a study area in its core distribution range (Extremadura, Spain). We evaluated annual and long-term variation in breeding parameters, as well as changes in environmental variables such as weather or harvest date. We then evaluated breeding failure and productivity in relation to nest protection measures, weather conditions, lay date and colony size. We found a significant trend for productivity to decrease over the 17-year study period, associated with a trend for predation probability to increase over time. Harvest occurred increasingly earlier in more recent years. The success rate of nests protected with unfenced unharvested patches (the most frequently used protection measure) increased with harvest date, but also declined throughout the study period for equivalent harvest dates. When considering all explanatory variables together, probability of nest success decreased significantly with lay date and throughout the study period, increased with annual rainfall and varied in relation to protective measures (being highest for nests protected with fences or where harvest in the plot had been delayed). In addition, among successful nests, fledged brood size also decreased significantly with lay date and temperature, and increased with annual rainfall. We found no effect of number of neighbours on breeding output. We discuss our results in relation to future conservation prospects for the species.
Zusammenfassung
Bruterfolg von Wiesenweihen in Abhängigkeit von Witterung, Koloniegröße und Schutzmaßnahmen: eine Langzeitstudie in der Extremadura, Spanien
Auf der Grundlage von Langzeitstudien (2001–2017) und umfangreichen Datensätzen von mehr als 1.700 Brutversuchen der Wiesenweihe (Circus pygargus) im Projektgebiet untersuchen wir Faktoren, welche den Bruterfolg dieses mittelgroßen, bodenbrütenden Greifvogels maßgeblich beeinflussen. Die Wiesenweihe hat einen bedeutenden Verbreitungsschwerpunkt in ‚La Serena‘, Extremadura, wo ihre Nester meist in losen Kolonieverbänden im Getreide zu finden sind. Wir haben den Einfluss der für einen Bruterfolg maßgeblichen Faktoren wie Wetterdaten und Erntezeitpunkt sowohl für die einzelnen Jahre wie auch über den gesamten Untersuchungszeitraum betrachtet. Anschließend haben wir den Einfluss der angewandten Schutzmaßnahmen, Witterungsverhältnisse, Legedatum und Koloniegröße auf Produktivität und Nestverluste untersucht. Über den gesamten Untersuchungszueitraum von 17 Jahren hinweg konnten wir eine signifikante Abnahme des Bruterfolges feststellen; gleichzeitig stieg die Wahrscheinlichkeit eines Nestverlusts aufgrund von Prädation. In den letzten Jahren begannen die Ernteaktivitäten immer früher. Ein später Erntezeitpunkt vergrößerte die Aussichten auf einen Bruterfolg für Nester, die nur mit einer (nicht umzäunten) kleinen Restfläche nicht geernteten Getreides (der am häufigsten angewendeten Schutzmaßnahme) geschützt wurden. Im Laufe der Jahre jedoch sank die Wahrscheinlichkeit auf einen Bruterfolg auch bei später Ernte aufgrund zunehmender Prädation. Insgesamt verringerte sich die Wahrscheinlichkeit für einen Bruterfolg signifikant während des gesamten Untersuchungszeitraums und mit einem späten Legebeginn. Höhere jährliche Niederschlagsmengen hatten einen positiven Einfluss auf den Bruterfolg. Dieser war außerdem stark abhängig von den durchgeführten Schutzmaßnahmen: die besten Erfolge wurden bei einem späten Erntezeitpunkt und durch Umzäunung der Nester erzielt. Bei Nestern, in denen mindestens ein Jungvogel flügge wurde, war die Anzahl der flüggen Jungvögel bei spätem Legebeginn signifikant niedriger, ebenso wirkten sich sehr hohe Temperaturen negativ auf die Anzahl der flüggen Jungvögel aus. Dagegen hatten hohe Niederschlagsmengen einen positiven Effekt auf die Anzahl der flüggen Jungvögel. Ein Zusammenhang zwischen Bruterfolg und Koloniegrößer (Anzahl benachbarter Brutpaare) konnte nicht nachgewiesen werden. Wir diskutieren unsere Ergebnisse im Hinblick auf weiterhin erforderliche Schutzmaßnahmen für diese bedrohte Art.
Similar content being viewed by others
Introduction
Knowledge about factors influencing breeding parameters is essential to understand population dynamics and to design management strategies for species of conservation concern. In general, both biotic and abiotic factors affect breeding in birds. Habitat quality is an essential factor affecting breeding in most species. In farmland areas, land use changes due to adjustments in agricultural markets, and modification and intensification in agricultural practices (increased efficiency of harvesting machines, use of pesticides or earlier-harvesting crop varieties) may strongly influence breeding, either through affecting availability of breeding habitat or prey, or directly through mortality or nest destruction (Donald et al. 2001; Robinson and Sutherland 2002). Timing of breeding is also frequently associated with differences in breeding success, with earlier-breeding individuals being more successful, either because earlier laying is associated with older age or higher individual quality, or because environmental conditions and food supply degrade throughout the breeding season (Newton and Marquiss 1984; Pietiäinen 1989; Meijer et al. 1990; Sæther 1990). Additionally, intraspecific relationships may influence breeding success for many species, and local breeding density has been described as affecting breeding parameters (e.g. Bertram 1978; Wittenberger and Hunt 1985; Siegel-Causey and Kharitonov 1990; Brown and Brown 1996). Among non-biotic factors, weather is a limiting factor for many species, and studying the relationships between weather and breeding is important, particularly in view of recent climate change (Moss et al. 2001; Carvalho et al. 2011). Finally, human intervention as part of conservation schemes may also influence breeding in managed species, and assessing this is also important in order to evaluate efficiency of use of conservation resources (Sutherland et al. 2004).
Montagu’s Harrier (Circus pygargus) is a medium-sized ground-nesting species. As such, predation by both mammalian and aerial predators is an important determinant of breeding success in many areas (Arroyo et al. 2004). The species originally inhabited seminatural habitats like grasslands, but also dunes, heatherfields, marshes and steppes (Clarke 1996). However, although natural habitats are still used in many areas, particularly in eastern countries (Terraube et al. 2010; Sokolov 2017; Bashta et al. 2017), the species switched to breeding in cereal crops in most of Western Europe in the second half of the twentieth century (Arroyo et al. 2004; Mebs and Schmidt 2006). When the species nests in crops, harvesting activities endanger the nestlings of Montagu’s Harrier if they are not yet able to fly at harvest time, and conservation campaigns to protect nests at harvest time occur in many areas of its breeding range (Arroyo et al. 2001; Kitowski 2002; Koks and Visser 2002; Santangeli et al. 2014, 2015; Illner 2017). It has been shown that efficiency of conservation measures depends on the relative time when harvest occurs in relation to the breeding cycle (Santangeli et al. 2014), so that early harvest leads more frequently to breeding failure even when conservation measures are applied. Additionally, it has also been shown that protecting against predation risk markedly increases the efficiency of protection at harvest time (Santangeli et al. 2015). The species is also semi-colonial, nesting in isolation or in loose colonies (Arroyo 1995). Semi-colonial nesting has been shown to have advantages in relation to anti-predator behaviour, including earlier predator detection and lower risk in predator deterrence (Arroyo et al. 2001), and has also been described to influence breeding parameters (Wiacek 2008; Kitowski 2008; Krupinski et al. 2010). At a European level, probability of occurrence of Montagu’s Harrier as a breeding bird increases with temperature, (Garcia and Arroyo 2001), suggesting that the species is adapted to the high temperatures found in southern latitudes. Indeed, no negative effect was found in central Spain between temperature and Montagu’s Harrier reproduction, in contrast to that observed in sympatric Hen Harriers Circus cyaneus (Garcia and Arroyo 2001). However, it has been forecasted that further increase in temperatures would reduce the environmental favourability of these areas for the species (Estrada et al. 2010), so the temperature increase observed in recent decades may affect reproduction.
On the basis of long-term (2001–2017) and extensive data (> 1700 breeding attempts) in a study area in the core distribution range (Extremadura, Spain), we assess factors influencing breeding parameters in this conservation-dependent species. We evaluate annual and long-term variation in breeding parameters, as well as changes in environmental variables such as weather or harvest date. We then evaluate breeding failure and productivity in relation to nest protection measures, weather conditions, lay date and colony size. In particular, we test the following hypotheses. (1) Earlier lay date should be correlated with higher breeding success. (2) In Mediterranean areas, higher rainfall is an indicator of primary productivity, and therefore higher vegetation development and prey abundance. Therefore, it should be advantageous to harrier breeding. (3) Very high temperatures (as observed sometimes in Southern Spain), in contrast, could be detrimental for breeding. (4) A larger number of neighbours should decrease predation risk. (5) The efficiency of different conservation measures may depend on predation risk or harvest date. We discuss our results in relation to the conservation status and perspectives for the species.
Methods
Study area and species
The breeding area in ‘La Serena’ covers around 350 km2, and is situated in the south eastern part of Extremadura (southwestern Spain) between the towns of Cabeza del Buey (coordinates 38.737778, − 5.219444), Castuera (coordinates 38.706389, − 5.544444) and Villanueva de la Serena (coordinates 38.973889, − 5.800278), at an altitude of 400–550 m above sea level. It is a flat landscape, occupied by a pseudo-steppe, where land use is mainly a mosaic of cereal, pasture and fallow land (representing the traditional way of farming, with pasture for extensive livestock, and arable land cultivated only every other year, to allow soil to recover between crops, half of arable land thus being fallow every year). Montagu’s Harriers find both breeding habitats (mainly in oat or barley) and hunting areas there.
The Montagu’s Harrier breeds from Western Europe to Asia, but a large part of the breeding population in Europe is located in the south-western Iberian Peninsula (including Extremadura). Average distance between nests is 202 ± 125 m (Arroyo et al. 2004), although denser situations have been described in the literature (Wiacek 2008; Kitowski 2008; Krupinski et al. 2010). Our study population represents ca. 15–20% of the Extremadura population as a whole. Montagu’s Harriers arrive in the breeding areas of Extremadura in March or early April, and onset of egg laying normally occurs between mid-April and mid-May (Arroyo et al. 2004) but can vary considerably within a year. Clutch size is usually 4–5 eggs, occasionally up to 6 eggs. Montagu’s Harriers are food generalists and feed on small mammals, small birds, reptiles and also insects; in Extremadura, the proportion of small birds and insects is large (Terraube and Arroyo 2011).
Conservation methods
A campaign for the protection of Montagu’s Harrier started in ‘La Serena’ in 1999. Volunteers from different countries have worked every year in the fields to locate as many nests as possible. Since 2001 the campaign has been organized by ANSER (Amigos de la Naturaleza de la Serena), the local non-governmental association for the protection of nature as part of the nationwide Iberian Working Group on Harriers (Garcia and Arroyo 2002). Where possible, negotiations with farmers to delay harvest date in fields with nests occurred to protect nests and fledglings during harvest time. In other cases, when harvesting took place, the drivers of the combines left an unharvested patch of 4 × 4 m around each nest, thus ensuring some protection for the nestlings and the breeding pairs (Arroyo et al. 2002). When the combine had not left a satisfactory patch, some straw was put around the nest to offer some rudimentary protection to the nestlings. After harvesting, shepherds often used the fields as grazing grounds for their sheep. When we knew that sheep would enter the fields, we put a fence (approx. 1.5 × 1.5 m) around the nest in order to protect it from being trampled. From 2015 nests were fenced when at least one chick had hatched, in response to an increased predation rate (see “Results”).
Field data collection
Intensity of field work increased over the years owing to the cooperation of more volunteers (Tables 1, 2), and their field experience also increased. Nests were located by observing the females landing at the nest site after a food pass. Located nests were visited and marked by fixing plastic stripes at vegetation stalks around the nest. Numbers of located pairs represent underestimates of the breeding population, in particular for the period 2001–2006; during that time only 2–4 volunteers were in the field, which was not enough to cover the whole area and obtain reliable data for the number of breeding pairs and total number of fledglings. From 2007 onwards there were 5–10 volunteers per season (Tables 1, 2), so a larger share of the nests could be located. Nevertheless, the drivers of combines generally found a few nests each year that had not been previously located. Thus, we estimate our data coverage to be around 80% after 2007, and lower before that.
For each located nest, we noted the following variables: (1) GPS coordinates, from which we calculated distance to the nearest neighbour and number of neighbours within 600 m (referring to the colony definition of Arroyo (1995)). (2) Clutch size. (3) Number of fledglings. For nests that failed, we also assessed causes of failure, as predation (when we found remains of eggshells, injured dead chicks or a suddenly empty nest), abandonment (if the nest was found without eggs during two consecutive visits, the second one also without a female or if we found the nest with eggs but no female), trampled by sheep, failed as result of harvesting, or other (e.g. when we found not injured dead chicks after extremely high temperatures, or nests failed for unknown cases). (4) Conservation method (fence, delayed harvest, 4 × 4 m patch without harvesting, other (including straw circle and transfer to a recovery station). (5) Harvesting date was noted for 1056 nests. (6) For a subsample of nests (n = 698), we also estimated hatching date (through visual screening of chicks’ age, using a photo-gallery of the different stages). Lay date for those nests was estimated as hatching date minus 30 days for incubation (Mebs and Schmidt 2006). (7) For a subsample of nests (n = 582), we also measured vegetation height at nest discovery, in centimetres.
Weather variables
In Mediterranean areas like Spain, precipitation levels during autumn, winter and spring are correlated with higher numbers of potential prey for Montagu’s Harriers, and dense vegetation for nest building (Soriguer 1981; Borralho et al. 1998; Garcia and Arroyo 2001). We obtained monthly precipitation in the study area for each study year, and from this we calculated the cumulative precipitation for the 9 months prior and partly including the breeding season. “Precipitation 2012”, for example, indicates cumulative precipitation from October 2011 to June 2012 (corresponding to the agronomic year, from seeding to end of crop growth). Additionally, we calculated the cumulative number of days during the breeding season (April to July) with maximum temperatures higher than 38 °C as an indicator of overtly high temperature. Data were taken from www.tutiempo.net, for the weather station of Talavera la Real (located ca. 150 km from the study area, but in the same general region and subjected to similar climate).
Camera installation
With the aim of evaluating predation risk and predators involved in more detail, we used trail cameras (Härting and Illner 2015) in 2016 and 2017 to monitor predation events. We installed five Dörr trail cameras (https://www.wildkamera.eu/doerr-snapshot-mobil/) (six in 2017) at a distance of approximately 1 m from the centre of the nest. Cameras were used for another nest after the chicks had fledged or the nest had failed. We thus monitored 10 nests in 2016 and 12 nests in 2017 during 3–30 days each. The cameras contained an integrated PIR (pyroelectric infrared) sensor that was capable of detecting rapid temperature changes caused by a moving animal. The cameras were programmed to take a series of two pictures whenever something was moving followed by an interval of at least 2 min. During the night, the camera activated an infrared flashlight. To avoid overexposure, part of the IR diodes had to be covered by opaque tape. The cameras also recorded time and temperature.
Statistical analyses
General linear models (GLMs) were used to analyse annual and temporal variation in breeding parameters, using information from each nest as a data point. Differences among years were tested by including year as a factor as an explanatory variable, whereas temporal trends were tested including year as a continuous variable as explanatory variable. In these models, lay date and harvest date were fitted to a normal distribution (with an identity link function), breeding success and predation probability were fitted to a binomial distribution (logit link function), fledged brood size (number of fledglings in successful nests) was fitted to a Poisson distribution (log link function), and productivity (number of fledglings per breeding pair) to a zero-inflated Poisson distribution (log link function). For the analyses of temporal trends in harvest dates, we excluded data from plots where a delay in harvest had been agreed with the farmers. Statistical software R 3.2.4 (R Core Team 2017) was used throughout, with the libraries nlme, lme4, effects.
Temporal trends in rainfall or temperature were tested with Pearson’s correlation (with n = 17 points, one per annual value). Pearson’s correlations were also used to assess the relationship between vegetation height and rainfall.
Efficiency of conservation measures was tested with a general linear mixed model (GLMM), using nest success as a response variable (binomial distribution, logit link function), and conservation measure (a categorical variable with five categories: “fenced” (nests), (harvest) “delay”, “buffer” (unharvested patches), “other” and “none”) as an explanatory variable. In this model (Fig. 3), “year” and “colony” were included as random factors, to account for annual variations in breeding success unrelated to conservation measures, and for potential differences among breeding territories (including the possibility of the same individuals breeding in the same territory among years). A GLMM was also used to evaluate temporal trends on nesting success for nests protected with unharvested patches. Here, “year” was included as an explanatory variable as a continuous variable, and as a categorical variable as a random term, together with “colony identification”. We also included harvest date as an explanatory variable.
GLMMs with year and colony as random factors were also used to analyse the additive effect of different variables affecting breeding output. Productivity (number of fledglings per breeding pair) had a zero-inflated distribution, and we could not fit zero-inflated mixed models. Therefore, we used GLMMs with year as a random factor to analyse variables affecting nest success and fledged brood size (number of fledglings produced at each successful nest) separately. The first one was fitted to a Poisson distribution (log link function), and the latter to a binomial distribution (logit link function). As explanatory variables we included lay date, annual rainfall, days with temperature higher than 38 °C, number of neighbours within 600 m, type of protection method and year as a continuous variable (Figs. 4, 5). For these analyses, year and rainfall were standardized (as its value minus the mean divided by the standard deviation) to help with model convergence. We also tested for two-way interactions, but they were never significant and results are not presented.
Results
Variation in breeding parameters
The number of nests monitored each year sharply increased after 2007 (coinciding with an increase in the number of volunteers, Tables 1, 2), and reached a maximum in 2011 (179 breeding pairs), from when located nests declined steadily with time despite similar searching effort (Tables 1, 2) reaching a minimum of 80 breeding pairs in 2016.
Average distance between nests was 131 ± 168 m (mean ± SD). Most (98%, n = 1707) nests occurred clumped in colonies (nearest neighbour < 600 m apart). We consistently found 1–2 large colonies (containing 15–45 breeding pairs accounting for about 35% of the population) each year, while the size of most other colonies ranged between 4 and 14 pairs. Spatial arrangement of nests within a given colony could be anything between compact and linear (see examples in Fig. 1). Therefore, number of neighbours within 600 m (and thus local density) varied strongly among nests, even within colonies, ranging from 1 to 35.
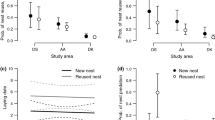
Most laying occurred in the second half of April, but laying occurred throughout an extended period, covering almost two months (Fig. 2a). Lay date varied significantly among years (Table 3), with almost two weeks difference among years (Tables 1, 2). We also found a significant trend for laying to occur increasingly later over the study period (Fig. 2b, Table 3).
a Frequency distribution of lay dates (1 = 1 April), the peak is 16 – 30 April. b Modelled relationship of lay date in relation to study year. Lay dates retarded up to 3 days over 17 years. See Table 3 for statistical results. c Modelled relationship of harvest date in relation to study year. Harvest date advanced by 7 days over 17 years. See Table 3 for statistical results. Error bars represent 95% CI
Average clutch size was 4.1 ± 0.9 (n = 941), varied significantly among years and did not change over the study period (Table 3). On the other hand, it decreased significantly with lay date (\(\chi_{ 1}^{ 2}\) = 8.88, P = 0.003, slope − 0.008 ± 0.003).
Productivity (fledglings/breeding pair) averaged 1.58 ± 1.65 (n = 1760). This parameter varied significantly among years, ranging from 0.32 ± 0.84 (in 2012) to 2.69 ± 1.41 (in 2003) (Tables 1, 2). We also found a significant trend for productivity to decrease with lay date (\(\chi_{1}^{2}\) = 24.43, P < 0.0001, slope − 0.012 ± 0.002), and over the study period (Table 3), passing from 2.15 ± 1.38 in the period 2001–2005 and 2.03 ± 1.62 in 2006–2010 to 1.10 ± 1.60 in 2011–2017.
Fledged brood size (fledglings/successful pair) averaged 2.97 ± 0.98 (n = 937), ranging among years from 2.24 ± 0.77 to 3.42 ± 0.90. Among-year differences were statistically significant, and we found a decline with lay date (\(\chi_{1}^{2}\) = 8.86, P = 0.003, slope − 0.007 ± 0.002), but there was no temporal trend among years (Table 3).
Of the monitored nests, 53% (n = 1760) failed before fledging. The main reason for nest failure was predation (76% of 621 failed nests), but we also recorded nest destruction by harvesting machines or nests being trampled by sheep. Nest success rate and predation rate varied significantly among years (Table 3). We also found a significant trend for success rate to decrease and predation rate to increase with lay date (\(\chi_{1}^{2}\) = 35.58, P < 0.0001, slope − 0.048 ± 0.008 and \(\chi_{1}^{2}\) = 33.99, P < 0.0001, slope − 0.049 ± 0.009 respectively) and throughout the study period (Table 3, Fig. 3). Predation rate of nests averaged 22% for the period 2001–2011 and 50% for the period 2012–2017. Overall, predation occurred after harvest in 56% of cases. However, the likelihood of predation occurring before harvest increased significantly throughout the study period (\(\chi_{1}^{2}\) = 11.21, P = 0.0008, slope 0.076 ± 0.023).
Modelled representation of GLMM analysis of the effect of intervention method (a) and harvest date (b) on success rate (proportion of nests where at least one fledgling survived). Late harvest dates are correlated with increasing success rate. See Table 3 for statistical results. Error bars represent 95% CI
Trail cameras showed that foxes were the main predators. None of the nests monitored with trail cameras in 2016 got predated during the observation period (although two of them were predated later on). On the other hand, of the 12 monitored nests in 2017, five were predated by a fox; two of them before harvest and three after harvest, all of them at night. One camera also documented an eagle owl predating the nest, also at night. Of the other six monitored nests, hatchlings died in two nests (during periods of very high temperatures, temperature reaching 49 °C at the nest according to the cameras), and in another one the female abandoned the nest with eggs, possibly also because of high temperatures. In the three remaining monitored nests, nestlings fledged.
Harvest date decreased significantly with year (Table 3), being on average 7 days earlier at the end compared to the beginning of the study period (Fig. 2c). Because lay date also was delayed over the years (Fig. 2b), the difference between harvest day and lay date decreased more than 8 days throughout the study period (\(\chi_{1}^{2}\) = 16.31, P < 0.0001, slope − 0.53 ± 0.13).
Weather and vegetation
Rainfall varied markedly among years, from less than 200 to almost 700 mm (Tables 1, 2), but no temporal trends were found (Pearson’s r = 0.06, n = 17, P > 0.10). Similarly, the number of days with temperatures higher than 38 °C varied widely among years from 3 to 24 (Tables 1, 2), but again no temporal trends were found (r = − 0.23, n = 17, P = 0.5).
Vegetation height was higher in more rainy years (Tables 1, 2, r = 0.675, n = 13, P < 0.01).
Protective interventions: comparison of techniques
Out of the 1758 monitored nests, 1355 were managed at harvest time (the remainder had either failed or, occasionally, fledged before harvest). Most nests (69%) were protected by leaving unharvested patches (buffers) around the nest. Additionally, 13% of nests were protected through fencing them, and 11% were managed by delaying harvest in the whole plot until 1 July (n = 65 nests) or until the end of summer (n = 86). The fabrication of a straw circle around the nestlings was also used in 86 nests, and nine were moved to a recovery centre (in figures, the two latter techniques are included in the category “other”).
The success rate of nests protected with unharvested patches increased with harvest date, but also declined throughout the study period for equivalent harvest dates (GLMM, \(\chi_{1}^{2}\) = 9.55, P = 0.002, slope 0.041 ± 0.013 for harvest date, and \(\chi_{1}^{2}\) = 35.09, P < 0.0001, slope − 1.44 ± 0.24 for year). In recent years, predation rate of nests protected with unharvested patches was very high (Tables 1, 2).
Even when taking into account harvest date, there were significant differences in overall success rate (proportion of protected nests where at least one fledgling was produced) among intervention methods (GLMM, \(\chi_{1}^{2}\) = 6.03, P = 0.01 for harvest date; \(\chi_{4}^{2}\) = 50.05, P < 0.0001 for intervention method), with success being higher for fencing the nest and delaying harvest in the plot (Fig. 3).
Factors affecting variation in breeding output
When considering all explanatory variables together (lay date, annual rainfall, days with temperature higher than 38 °C, number of neighbours within 600 m, type of protection method and year), probability of nest success decreased significantly with lay date and throughout the study period, increased with annual rainfall and varied in relation to protective measures (Table 4, Fig. 4). Neither number of neighbours nor temperature had a significant effect on nest success.
Modelled representation of the GLMM analysis of the relationship between nest success (proportion of nests with at least one surviving fledgling) and explanatory variables. See Table 4 for statistical results. Error bars represent 95% CI. a Effect of colony size (number of neighbours < 600 m, abscissa) on nest success (ordinate), the effect is not significant. b Increasing values of lay date have a negative effect on nest success. c Increasing values of rainfall have a positive effect on nest success. d The number of days with temperatures > 38 °C over the years had no significant effect on nest success. e Relationship between nest success and protection types “fenced” and “delay” had the strongest positive effect on nest success. f Over the years of this study, nest success decreased significantly
Among successful nests, fledged brood size decreased significantly with lay date and increased with annual rainfall. Additionally, and in contrast to nest success, temperature did have a significant influence on fledged brood size, with lower number of fledglings in successful nests with increasing temperature. No other variables had a significant effect on fledged brood size (Table 4, Fig. 5).
Modelled representation of the GLMM analysis of fledged brood size (number of fledglings per successful nest) and explanatory variables. See Table 4 for statistical results. Error bars represent 95% CI. Relationship between fledged brood size (ordinate) and a colony size (number of neighbours < 600 m, abscissa), b lay date, c rainfall, d days with temperatures > 38 °C, e protection type, f year
Discussion
As in many other species (Newton and Marquiss 1984; Pietiäinen 1989; Meijer et al. 1990; Sæther 1990), we found a strong relationship between lay date and breeding output, with productivity being lower for later-breeding pairs. Older (and more experienced) Montagu’s Harrier females are known to breed earlier, lay larger clutches and produce more fledglings than younger females (Arroyo et al. 2007), which may explain these differences. Also, later-breeding birds may be more exposed to harvesting problems, and environmental conditions may degrade throughout the breeding season, as suggested by the fact that nest failure probability and predation probability increased for later-breeding pairs. For example, food abundance or accessibility may be lower later in the season, higher temperatures may lead to lower useful time for foraging, and nests may be more visible for predators after harvest has started.
Additionally, we found a significant degradation of breeding success throughout the 17-year study period, associated with a strongly reduced efficacy of the most common protection method (buffers). The fact that neither clutch size nor fledged brood size decreased with time suggests that factors such as food abundance at the beginning of the breeding season (probably modulated in this Mediterranean area by rainfall levels in winter and spring), driving clutch size, have not changed, and that what has changed with time are conditions later in the breeding season that have led to higher nest failure. This degradation is at least partly associated with earlier harvest dates in recent times (Fig. 2c), and increased predation rate. Earlier harvest may be associated with different cereal varieties being used, a change in weather conditions, or other factors. With current information it is not possible to determine the ultimate driver of earlier harvest, but the observed advance of harvest date with time is highly relevant as it has a significant effect on efficacy of conservation methods for this vulnerable species (Santangeli et al. 2014), as well as on breeding success for many other ground-nesting species (e.g. Casas and Viñuela 2010). The observed increase in predation rates may also be facilitated by the earlier harvest dates (and thus higher vulnerability of nests after harvest). However, the fact that the probability of predation before harvest also increased over the study period indicates the potential occurrence of other factors, such as increased predator population over the years. Field observations indeed support increased number of foxes in the area (B. Berger-Geiger, pers. obs.). In any case, the higher nest failure rate leading to lower overall productivity may have led to the observed decline in population size in recent years; indeed, the productivity observed in recent years (averaging just above one fledgling per breeding pair) is known to be insufficient for population sustainability (Arroyo et al. 2002). Conditions observed in our study area may reflect what is happening in that area at large, as a similar decreasing population trend has been found for the whole of the Extremadura region (Seo/Birdlife 2018).
Our results also have implications for conservation management: highest success was overall achieved with delayed harvest and fenced nests, and in fact success of nests protected with unfenced buffers was too low in recent years. A similar situation was found in Germany, where success of unfenced large buffers (50 × 50 m) has also decreased with time (https://www.lbv.de/naturschutz/artenschutz/voegel/wiesenweihe/aktuelles-zur-wiesenweihe-in-bayern/). Our results thus indicate that future interventions in the area should favour nest fencing or harvest delay when possible. Nevertheless, delaying harvest in many of the fields with harrier nests may be problematic because of both economic costs and agronomic limitations (as most farmers in our study region depend on contracted harvesting machines coming from other areas). Fencing nests could be a cost-efficient and more easily implemented measure to protect harriers. This measure is efficiently used in France (Santangeli et al. 2015), the Netherlands (Koks and Visser 2002) and recently in Germany (reference above). Our data therefore strongly suggest that it would be essential for governments or conservation non-governmental organisation (NGOs) in the Iberian Peninsula to invest in fences to ensure breeding success of this vulnerable species in the study area. As the fences are retrieved and reused year after year, the material costs would occur only at the beginning.
Previous studies (Arroyo et al. 2001; Kitowski 2008; Krupinski et al. 2010) have suggested that breeding in colonies might be beneficial, even though in some parts of the breeding area Montagu’s Harrier do not live in colonies. Here, and in contrast to our predictions, we found no effect of number of neighbours on breeding success: larger colonies did not reduce their predation risk. This may be related to the fact that, in the trail cameras, all documented predation events (6 in 12 monitored nests) occurred at night, when communal defence against predators was not active. It is therefore possible that communal defence within a colony works well to reduce predation risk during daytime (e.g. against raptors, corvids or white storks), but that this potential benefit of coloniality disappears when predation is mostly nocturnal.
Finally, we found a significant effect of weather on breeding parameters, according to our predictions. Higher rainfall levels in winter and spring were associated with higher success probability and fledged brood size. In Mediterranean areas, winter and spring rainfall is associated with higher primary production and, indeed, we found an association between rainfall and higher vegetation (so probably reducing predation risk). But, additionally, higher rainfall and primary production is also associated there with higher prey abundance (e.g. Herrera 1980; Soriguer 1981; Lucio 1990; Tellería 1996; Borralho et al. 1998), which may explain differences in fledged brood size. On the other hand, very high temperatures (up to 49 °C) were associated with smaller fledged brood size, and this probably reflects a higher probability of nestlings dying of overheat (which was also observed in the trail cameras). Climate change predictions for the area indicate higher aridity and higher temperatures in the future. Therefore, environmental favourability of this area may decrease in the future (Estrada et al. 2010), and breeding success may further decrease under those conditions. Therefore, the conservation status of Montagu’s Harrier may become more fragile in the future, and conservation efforts should be intensified to evaluate protection regimes, suitable for a changing environment in the Spanish Extremadura and elsewhere.
References
Arroyo B (1995) Breeding ecology and nest dispersion of Montagu’s harrier Circus pygargus in central Spain. PhD thesis, Univ. Oxford
Arroyo B, Mougeot F, Bretagnolle V (2001) Colonial breeding and nest defence in Montagu’s harrier (Circus pygargus). Behav Ecol Sociobiol 50(2):109–115
Arroyo B, Garcia JT, Bretagnolle V (2002) Conservation of the Montagu’s harrier (Circus pygargus) in agricultural areas. Anim Conserv 5:283–290
Arroyo B, García JT, Bretagnolle V (2004) Circus pygargus Montagu’s Harrier. BWP Update 6(1 & 2):39–53
Arroyo BE, Bretagnolle V, Leroux A (2007) Interactive effects of food and age on breeding in the Montagu’s Harrier Circus pygargus. Ibis 149(4):806–813
Bashta A-T, Domashevsky S, Vetrov V, Skrypan M (2017) The status of the Montagu’s harrier Circus pygargus in Ukraine. Vogelwelt 137:366–371
Bertram BCR (1978) Living in groups: predators and prey. In: Krebs JR, Davies NB (eds) Behavioural ecology: an evolutionary approach. Blackwell, Oxford, p 221–248
Borralho R, Rito A, Rego F, Simoes H, Pinto PV (1998) Summer distribution of Red-legged Partridges Alectoris rufa in relation to water availability on Mediterranean farmland. Ibis 140(4):620–625
Brown CR, Brown MB (1996) Coloniality in the cliff swallows. University of Chicago Press, Chicago
Carvalho SB, Brito JC, Crespo EG, Watts ME, Possingham HP (2011) Conservation planning under climate change: toward accounting for uncertainty in predicted species distributions to increase confidence in conservation investments in space and time. Biol Conserv 144:2020–2030
Casas F, Viñuela J (2010) Agricultural practices or game management: which is the key to improve red-legged partridge nesting success in agricultural landscapes? Environ Conserv 37:177–186
Clarke R (1996) Montagu’s harrier. Arlequin, Chelmsford
Donald PF, Green RE, Heath MF (2001) Agricultural intensification and the collapse of Europe’s farmland bird populations. Proc R Soc Lond Ser B Biol Sci 268(1462):25–29
Estrada A, Arroyo B, Márquez A (2010) Possible changes in favourability areas for Montagu’s and hen harriers in Spain according to climate change scenarios. Ardeola 57:123–128
Garcia JT, Arroyo BE (2001) Effect of abiotic factors on reproduction in the centre and periphery of breeding ranges: a comparative analysis in sympatric harriers. Ecography 24(4):393–402
Garcia JT, Arroyo B (2002) Population trends and conservation of Montagu’s Harrier in Spain. Orn. Anz. 41:183–190
Härting C, Illner H (2015) Kameraüberwachung von Nestern der Wiesenweihe zur Abschätzung des Einflusses von Prädatoren. ABU info 36–38:14–26
Herrera CM (1980) Composición y estructura de dos comunidades mediterraneas de passeriformes. Doñana Acta Vertebrata 7:1–340
Illner H (2017) Brutbestände der Wiesenweihe Circus pygargus und Nestschutzmaßnahmen in Deutschland 2003 bis 2014. Vogelwelt 137:305–317
Kitowski I (2002) Present status and conservation problems of Montagu’s Harrier Circus pygargus in Southeast Poland. Orn. Anz. 41:167–174
Kitowski I (2008) Breeding ecology of Montagu’s Harrier (Circus Pygargus) in marshes of Eastern Poland: importance of aggregated nesting. Acta Zool Lit 18(2):83–89
Koks B, Visser EG (2002) Montagu’s Harriers Circus pygargus in the Netherlands: does nest protection prevent extinction? Orn Anz 41:159–166
Krupinski D, Lewtak J, Szulak K (2010) Semicolonial nesting and conservation of the Montagu’s harrier Circus pygargus in rapeseed fields in Southern Podlasie (eastern Poland). Slovak Raptor J 4:37–40
Lucio AJ (1990) Influencia de las condiciones climaticas en la productividad de la perdiz roja (Alectoris rufa). Ardeola 37:207–218
Mebs T, Schmidt D (2006) Die Greifvögel Europas, Nordafrikas. Franckh-Kosmos Verlag, Stuttgart, Vorderasiens
Meijer T, Daan S, Hall M (1990) Family planning in the kestrel (Falco tinnunculus): the proximate control of covariation of laying date and clutch size. Behaviour 114:117–136
Moss R, Oswald J, Baines D (2001) Climate change and breeding success: decline of the capercaillie in Scotland. J Anim Ecol 70:47–61
Newton I, Marquiss M (1984) Seasonal trend in the breeding performance of sparrowhawks. J Anim Ecol 53:809–830
Pietiäinen H (1989) Seasonal and individual variation in the production of offspring in the Ural owl Strix uralensis. J Anim Ecol 58:905–920
R Core Team (2017) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/. Accessed 17 Dec 2018
Robinson RA, Sutherland WJ (2002) Post-war changes in arable farming and biodiversity in Great Britain. J Appl Ecol 39(1):157–176
Sæther BE (1990) Age-specific variation in reproductive performance of birds. Cur Ornithol 7:252–293
Santangeli A, Di Minin E, Arroyo B (2014) Bridging the research implementation gap – Identifying cost-effective protection measures for Montagu’s harrier nests in Spanish farmlands. Biol Conserv 177:126–133
Santangeli A, Arroyo B, Millon A, Bretagnolle V (2015) Identifying effective actions to guide volunteer-based and nationwide conservation efforts for a ground-nesting farmland bird. J Appl Ecol 52(4):1082–1091
Seo/BirdLife (2018) Descienden las poblaciones de aguilucho cenizo y aguilucho pálido en España en los últimos 10 años. https://www.seo.org/2018/07/06/descienden-las-poblaciones-de-aguilucho-cenizo-y-aguilucho-palido-en-espana-en-los-ultimos-10-anos/. Accessed 17 Dec 2018
Siegel-Causey D, Kharitonov SP (1990) The evolution of coloniality. Curr Ornithol 7:285–330
Sokolov A (2017) Montagu’s harrier Circus pygargus in European Russia: current status, dynamics of breeding range and numbers, some ecological aspects. Vogelwelt 137:359–365
Soriguer RC (1981) Biología y dinamica de una poblacion de conejos (Oryctolagus cuniculus L.) en Andalucía occidental. Doñana Acta Vertebrata 8:1–379
Sutherland WJ, Pullin AS, Dolman PM, Knight TM (2004) The need for evidence-based conservation. Trends Ecol Evol 19:305–308
Tellería JL (1996) Estepas y comunidades de aves. In: Fernandez Gutierrez J, Sanz-Zuasti J (eds) Conservacion de las aves esteparias y su habitat. Junta de Castilla y Leon, Valladolid, pp 19–25
Terraube J, Arroyo B (2011) Factors influencing diet variation in a generalist predator across its range distribution. Biodivers Conserv 20(10):2111–2131
Terraube J, Arroyo BE, Mougeot F, Katzner T, Bragin E (2010) Breeding biology of the Montagu’s harrier (Circus pygargus) in north-central Kazakhstan. J Ornithol 151:713–722
Wiacek J (2008) Benefits and costs of semi-colonial breeding in the Montagu’s Harrier Circus pygargus. Belgian J Zool 138(1):36–40
Wittenberger JF, Hunt GL (1985) The adaptive significance of coloniality in birds. In: Farner DS, King JR, Parkes KC (eds) Avian biology. Academic, New York
Acknowledgements
Many volunteers helped with fieldwork localising nests. Special thanks to Manolo Calderón, president of ANSER (Amigos de la Naturaleza en la Serena) who was responsible for funding and realizing the annual campaigns. Trail cameras were supported by Michael Wink, University of Heidelberg. Tobias Müller helped with the figures of the manuscript and Ingmar Geiger with preparing the immense amount of data for statistical analyses. This work complies with the current environmental laws in Spain.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Data availability
The datasets analysed during the current study are available from the corresponding author on reasonable request.
Additional information
Communicated by O. Krüger.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Berger-Geiger, B., Galizia, C.G. & Arroyo, B. Montagu’s Harrier breeding parameters in relation to weather, colony size and nest protection schemes: a long-term study in Extremadura, Spain. J Ornithol 160, 429–441 (2019). https://doi.org/10.1007/s10336-018-1618-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10336-018-1618-0