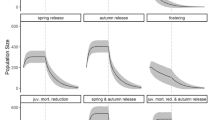
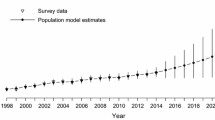
Abstract
The release of animals to reestablish an extirpated population is a decision problem that is often attended by considerable uncertainty about the probability of success. Annual releases of captive-reared juvenile Whooping Cranes (Grus americana) were begun in 1993 in central Florida, USA, to establish a breeding, non-migratory population. Over a 12-year period, 286 birds were released, but by 2004, the introduced flock had produced only four wild-fledged birds. Consequently, releases were halted over managers’ concerns about the performance of the released flock and uncertainty about the efficacy of further releases. We used data on marked, released birds to develop predictive models for addressing whether releases should be resumed, and if so, under what schedule. To examine the outcome of different release scenarios, we simulated the survival and productivity of individual female birds under a baseline model that recognized age and breeding-class structure and which incorporated empirically estimated stochastic elements. As data on wild-fledged birds from captive-reared parents were sparse, a key uncertainty that confronts release decision-making is whether captive-reared birds and their offspring share the same vital rates. Therefore, we used data on the only population of wild Whooping Cranes in existence to construct two alternatives to the baseline model. The probability of population persistence was highly sensitive to the choice of these three models. Under the baseline model, extirpation of the population was nearly certain under any scenario of resumed releases. In contrast, the model based on estimates from wild birds projected a high probability of persistence under any release scenario, including cessation of releases. Therefore, belief in either of these models suggests that further releases are an ineffective use of resources. In the third model, which simulated a population Allee effect, population persistence was sensitive to the release decision: high persistence probability was achieved only through the release of more birds, whereas extirpation was highly probable with cessation of releases. Despite substantial investment of time and effort in the release program, evidence collected to date does not favor one model over another; therefore, any decision about further releases must be made under considerable biological uncertainty. However, given an assignment of credibility weight to each model, a best, informed decision about releases can be made under uncertainty. Furthermore, if managers can periodically revisit the release decision and collect monitoring data to further inform the models, then managers have a basis for confronting uncertainty and adaptively managing releases through time.



Similar content being viewed by others
References
Akçakaya HR, Sjögren-Gulve P (2000) Population viability analyses in conservation planning: an overview. Ecol Bull 48:9–21
Akçakaya HR, McCarthy MA, Pearce JL (1995) Linking landscape data with population viability analysis: management options for the helmeted honeyeater (Lichenostomus melanopsis cassidix). Biol Conserv 73:169–176
Armstrong DP, Ewen JG (2001) Assessing the value of follow-up translocations: a case study using New Zealand robins. Biol Conserv 101:239–247
Beissinger SR, Westphal MI (1998) On the use of demographic models of population viability in endangered species management. J Wildl Manag 62:821–841
Bell TJ, Bowles ML, McEachern AK (2003) Projecting the success of plant population restoration with viability analysis. In: Brigham CA, Schwartz MW (eds) Population viability in plants: conservation, management, and modeling of rare plants. Springer, Berlin, pp 313–348
Binkley CS, Miller RS (1980) Survivorship of the Whooping Crane, Grus americana. Ecology 61:434–437
Buner F, Schaub M (2008) How do different releasing techniques affect the survival of reintroduced grey partidges Perdix perdix? Wildl Biol 14:26–35
Clark JS, Bjørnstad ON (2004) Population time series: process variability, observation errors, missing values, lags, and hidden states. Ecology 85:3140–3150
Courchamp F, Clutton-Brock T, Grenfell B (1999) Inverse density dependence and the Allee effect. Trends Ecol Evol 14:405–410
Deredec A, Courchamp F (2007) Importance of the Allee effect for reintroductions. Ecoscience 14:440–451
Ewen JG, Armstrong DP (2007) Strategic monitoring of reintroductions in ecological restoration programmes. Ecoscience 14:401–409
Falk DA (1992) From conservation biology to conservation practice: strategies for protecting plant diversity. In: Fiedler PL, Jain SK (eds) Conservation biology: the theory and practice of nature conservation, preservation, and management. Chapman and Hall, New York, pp 398–431
Fischer J, Lindenmayer DB (2000) An assessment of the published results of animal relocations. Biol Conserv 96:1–11
Folk MJ, Nesbitt SA, Parker JM, Spalding MG, Baynes SB, Candelora KL (2008) Current status of nonmigratory whooping cranes in Florida. Proc N Am Crane Workshop 10:7–12
Gilks WR, Thomas A, Spiegelhalter DJ (1994) A language and program for complex Bayesian modelling. Stat 43:169–177
Gilpin ME, Soulé ME (1986) Minimum viable populations: processes of species extinction. In: Soulé ME (ed) Conservation biology: the science of scarcity and diversity. Sinauer, Sunderland, pp 19–34
Heath SR, Kershner EL, Cooper DM, Lynn S, Turner JM, Warnock N, Farabaugh S, Brock K, Garcelon DK (2008) Rodent control and food supplementation increase productivity of endangered San Clemente Loggerhead Shrikes (Lanius ludovicianus mearnsi). Biol Conserv 141:2506–2515
Kleiman DV (1989) Reintroduction of captive mammals for conservation. Bioscience 39:152–161
Le Gouar P, Robert A, Choisy JP, Henriquet S, Lecuyer P, Tessier C, Sarrazin F (2008) Roles of survival and dispersal in reintroduction success of Griffon Vulture (Gyps fulvus). Ecol Appl 18:859–872
Link WA, Royle JA, Hatfield JS (2003) Demographic analysis from summaries of an age-structured population. Biometrics 59:778–785
McCarthy MA, Andelman SJ, Possingham HP (2003) Reliability of relative predictions in population viability analysis. Conserv Biol 17:982–989
Melbourne BA, Hastings A (2008) Extinction risk depends strongly on factors contributing to stochasticity. Nature 454:100–103
Menges ES (1991) The application of minimum viable population theory to plants. In: Falk DA, Holsinger KE (eds) Genetics and conservation of rare plants. Oxford University Press, New York, pp 45–61
Menges ES (1992) Stochastic modeling of extinction in plant populations. In: Fiedler PL, Jain SK (eds) Conservation biology: the theory and practice of nature conservation, preservation, and management. Chapman and Hall, New York, pp 253–275
Palmer MA, Falk DA, Zedler JB (2006) Ecological theory and restoration ecology. In: Falk DA, Palmer MA, Zedler JB (eds) Foundations of restoration ecology. Island Press, Washington D.C., pp 1–10
Pascual MA, Kareiva P, Hilborn R (1997) The influence of model structure on conclusions about the viability and harvesting of Serengeti wildebeest. Conserv Biol 11:966–976
Pavlik BM (1994) Demographic monitoring and the recovery of endangered plants. In: Bowles ML, Whelan CJ (eds) Restoration of endangered species: conceptual issues, planning, and implementation. Cambridge University Press, Cambridge, pp 322–350
Regan HM, Colyvan M, Burgman MA (2002) A taxonomy and treatment of uncertainty for ecology and conservation biology. Ecol Appl 12:618–628
Roche EA, Cuthbert FJ, Arnold TW (2008) Relative fitness of wild and captive-reared piping plovers: does egg salvage contribute to recovery of the endangered Great Lakes population? Biol Conserv 141:3079–3088
Runge MC, Johnson FA (2002) The importance of functional form in optimal control solutions of problems in population dynamics. Ecology 83:1357–1371
Sarrazin F, Barbault R (1996) Reintroduction: challenges and lessons for basic ecology. Trends Ecol Evol 11:474–478
Sarrazin F, Legendre S (2000) Demographic approach to releasing adults versus young in reintroductions. Conserv Biol 14:488–500
SAS Institute Inc (2004) SAS/IML 9.1 user’s guide. SAS Institute, Cary
Schaub M, Zink R, Beissmann H, Sarrazin F, Arlettaz R (2009) When to end releases in reintroduction programmes: demographic rates and population viability analysis of bearded vultures in the Alps. J Appl Ecol 46:92–100
Slotta-Bachmayr L, Boegel R, Kaczsensky P, Stauffer C, Walzer C (2004) Use of population viability analysis to identify management priorities and success in reintroducing Przewalski’s horses to southwestern Mongolia. J Wildl Manag 68:790–798
Stephens PA, Sutherland WJ (1999) Consequences of the Allee effect for behaviour, ecology and conservation. Trends Ecol Evol 14:401–405
Stephens PA, Sutherland WJ, Freckleton RP (1999) What is the Allee effect? Oikos 87:185–190
Taylor BL, Wade PR, Ramakrishnan U, Gilpin M, Akçakaya HR (2002) Incorporating uncertainty in population viability analyses for the purpose of classifying species by risk. In: Beissinger SR, McCullough DR (eds) Population viability analysis. University of Chicago Press, Chicago, pp 239–252
Wade PR (2002) Bayesian population viability analysis. In: Beissinger SR, McCullough DR (eds) Population viability analysis. University of Chicago Press, Chicago, pp 213–238
Wakamiya SM, Roy CL (2009) Use of monitoring data and population viability analysis to inform reintroduction decisions: peregrine falcons in the Midwestern United States. Biol Conserv 142:1767–1776
Williams BK, Nichols JD, Conroy MJ (2002) Analysis and management of animal populations. Academic Press, San Diego
Wilson AC, Stanley Price MR (1994) Reintroduction as a reason for captive breeding. In: Olney PJS, Mace GM, Feistner ATC (eds) Creative conservation: interactive management of wild and captive animals. Chapman and Hall, London, pp 243–264
Wolf CM, Griffith B, Reed C, Temple SA (1996) Avian and mammalian translocations: update and reanalysis of 1987 survey data. Conserv Biol 10:1142–1154
Acknowledgments
We thank the Florida Fish and Wildlife Conservation Commission and the International Whooping Crane Recovery Team for logistical support and guidance throughout our work. We also thank W.A. Link and J.S. Hatfield for helpful discussions early in our work, and we appreciate thoughtful manuscript reviews provided by S.K. Jacobi, L.A. Powell, and two anonymous reviewers. R. Boughton, B. Brooks, J.B. French, T.E. O’Meara, M. Putnam, J. Rodgers, and M. Spalding were members of a decision structuring team who provided input on selection of decision alternatives for simulation. Funding for our work was provided by the Wildlife: Terrestrial and Endangered Resources Program of USGS and by Patuxent Wildlife Research Center. Funding for the work by the FWC was supported in part by the U.S. Fish and Wildlife Service via Cooperative Agreement No. 401814-J-035. Finally, we thank the many employees and volunteers of the FWC who spent thousands of hours collecting the data that made our work possible. The use of trade, product, or company names does not imply endorsement by the U.S. Government. This work complied with applicable, current laws of the governments of the United States and the State of Florida.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by M. Schaub.
Appendix
Appendix
The statistical estimation models for survival and breeding class transition are described in the text. The accompanying mathematical descriptions are provided below and are more fully developed in Moore et al. (in preparation). Estimates from the models (Table 3) were used to construct the baseline PVA model.
Survival estimation
Each female bird j released into the wild as part of cohort c was a member of age or breeding class k (k ∈ {0, 1, 2, 3, 4 + , P, N, F}) in period i (3-month divisions of the year, starting from January 1993). The number of days that bird j survived within period i was assumed to be binomially distributed with probability \({ \text{p}_{ij}}(k,c)^{{1/d}_{i}} \) over x ij exposure days, where period i is d i days in length and 0 ≤ x ij ≤ d i.
Quarterly survival probability was modeled as a linear function of a mean (an intercept plus a female fixed effect) and random effects of age/breeding class, time, cohort, and individual:
where δ Gk is a random effect due to membership in age or breeding class k, δ Ti is a random effect due to period i, δ C*c are age-class specific random effects due to membership in release cohort c, δ B*j are age-class specific random effects due to bird j, and I(z) is the indicator function for expression z. Random effects were modeled as deviates from zero-centered normal distributions with corresponding variance parameters σ 2G , σ 2T , σ 2C0 , σ 2C1 , σ 2C2+ , σ 2B0 , σ 2B1 , and σ 2B2+ .
Posterior distributions of annual age and breeding-class specific survival rates (φk) were estimated by summing appropriate terms of the model above, transforming the sum to the probability scale, and multiplying four of the resulting terms together:
Estimation of productivity and breeding class transition probabilities
Transition into breeding class P occurred when a female bird of age ≥2 years first exhibited pairing behavior. For never-paired bird j that was age k (k = 1, 2, 3, 4+) in the year previous to i, we assumed that first pairing occurred as a Bernoulli outcome with probability θ UPij (k). Transition probability was thus modeled as a linear function of fixed intercept (μUP) and age-specific (βk) effects and of random effects of time (δ PTi ) and bird (δ PBj ):
Random effects were modeled as deviates from zero-centered normal distributions with corresponding variance parameters σ 2PT and σ 2PB . We obtained posterior distributions of annual age-specific transition probability into class P (ψ (k)UP ) by transformation of the above sum.
Transition from class P into class N occurred when a female produced her first nestling but failed to produce a fledgling. Transition from class P into class F occurred when a female’s first nestling developed into a fledgling (male or female). For bird j belonging to class P, both events were assumed to occur as Bernoulli outcomes with probabilities θ PNj and θ PFj , respectively. Linear models relating each transition probability to a fixed intercept and a random effect due to bird were:
Random effects were modeled as deviates from zero-centered normal distributions with corresponding variance parameters σ 2PNB and σ 2PFB . We obtained posterior distributions of annual transition probability from class P into classes N (ψPN) or F (ψPF). As no Florida bird has ever produced more than one fledgling in a year, ψPF serves as the estimate of productivity for birds in class P.
Transition from class N into class F occurred when a female who had only ever produced a nestling in prior attempts produced her first fledgling (male or female). We assumed that this event was a Bernoulli outcome with probability ψNF. Similarly, a bird already in class F produces another fledgling with probability ψFF. We modeled each of these probabilities as a simple mean with no random effects:
These probabilities effectively serve as estimates of productivity for birds in class N and F, respectively.
Rights and permissions
About this article
Cite this article
Moore, C.T., Converse, S.J., Folk, M.J. et al. Evaluating release alternatives for a long-lived bird species under uncertainty about long-term demographic rates. J Ornithol 152 (Suppl 2), 339–353 (2012). https://doi.org/10.1007/s10336-010-0592-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10336-010-0592-y