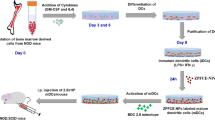
Abstract
Objective
Tracking the autoreactive T-cell migration in the pancreatic region after labeling with fluorinated nanoparticles (1,2-dioleoyl-sn-glycero-3-phosphoethanolamine-N-[3-(2-pyridyldithio)propionate]-perfluoro-15-crown-5-ether nanoparticles, PDP-PFCE NPs) in a diabetic murine model using 19F MRI.
Materials and methods
Synthesis of novel PDP-PFCE fluorine tracer was performed for in vitro labeling of T cells. Labeling conditions were optimized using different PDP-PFCE NPs concentrations. For in vivo 19F MRI, mice were longitudinally followed after adoptive transfer of activated, autoreactive, labeled T cells in NOD.SCID mice.
Results
Established MR protocols were used for challenging T cell labeling to track inflammation in a model of diabetes after successful labeling of CD4+ and CD8+ T cells with PDP-PFCE NPs. However, T cells were difficult to be detected in vivo after their engraftment in animals.
Discussion
We showed successful in vitro labeling of T cells using novel fluorinated liposomal nanoparticles. However, insufficient and slow accumulation of labeled T cells and subsequent T cell proliferation in the pancreatic region remains as limitations of in vivo cell imaging by 19F MRI.






Similar content being viewed by others
References
Liang S, Louchami K, Kolster H, Jacobsen A, Zhang Y, Thimm J, Sener A, Thiem J, Malaisse W, Dresselaers T, Himmelreich U (2016) In vivo and ex vivo 19-fluorine magnetic resonance imaging and spectroscopy of beta-cells and pancreatic islets using GLUT-2 specific contrast agents. Contrast Media Mol Imaging 11:506–513. https://doi.org/10.1002/cmmi.1712
Kriz J, Jirak D, Berkova Z, Herynek V, Lodererova A, Girman P, Habart D, Hajek M, Saudek F (2012) Detection of pancreatic islet allograft impairment in advance of functional failure using magnetic resonance imaging. Transpl Int 25:250–260. https://doi.org/10.1111/j.1432-2277.2011.01403.x
Malosio ML, Esposito A, Brigatti C, Palmisano A, Piemonti L, Nano R, Maffi P, De Cobelli F, Del Maschio A, Secchi A (2015) MR imaging monitoring of iron labeled pancreatic islets in a small series of patients: islets fate in successful, unsuccessful and auto-transplantation. Cell Transplant 24:2285–2296. https://doi.org/10.3727/096368914X684060
Medarova Z, Moore A (2008) Non-invasive detection of transplanted pancreatic islets. Diabetes Obes Metab 10(Suppl 4):88–97. https://doi.org/10.1111/j.1463-1326.2008.00942.x
Arifin DR, Bulte JWM (2011) Imaging of pancreatic islet cells. Diabetes Metab Res Rev 27:761–766. https://doi.org/10.1002/dmrr.1248
Alanentalo T, Asayesh A, Morrison H, Lorén CE, Holmberg D, Sharpe J, Ahlgren U (2007) Tomographic molecular imaging and 3D quantification within adult mouse organs. Nat Methods 4:31–33. https://doi.org/10.1038/nmeth985
Alanentalo T, Lorén CE, Larefalk A, Sharpe J, Holmberg D, Ahlgren U (2008) High-resolution three-dimensional imaging of islet-infiltrate interactions based on optical projection tomography assessments of the intact adult mouse pancreas. J Biomed Opt 13:054070. https://doi.org/10.1117/1.3000430
Peterson JD, Haskins K (1996) Transfer of diabetes in the NOD-scid mouse by CD4 T-cell clones: differential requirement for CD8 T-cells. Diabetes 45:328–336
Wucherpfennig KW, Eisenbarth GS (2001) Type 1 diabetes. Nature 2:767–768. https://doi.org/10.1016/S0140-6736(11)60614-4
Srinivas M, Boehm-Sturm P, Figdor CG, de Vries IJ, Hoehn M (2012) Labeling cells for in vivo tracking using 19F MRI. Biomaterials 33:8830–8840. https://doi.org/10.1016/j.biomaterials.2012.08.048
Himmelreich U, Hoehn M (2008) Stem cell labeling for magnetic resonance imaging. Minim Invasive Ther Allied Technol 17:132–142. https://doi.org/10.1080/13645700801969873
Himmelreich U, Dresselaers T (2009) Cell labeling and tracking for experimental models using Magnetic Resonance Imaging. Methods 48:112–124. https://doi.org/10.1016/j.ymeth.2009.03.020
Srinivas M, Heerschap A, Ahrens ET, Figdor CG, de Vries IJM (2010) 19F MRI for quantitative in vivo cell tracking. Trends Biotechnol 28:363–370. https://doi.org/10.1016/j.tibtech.2010.04.002
Harms C, Datwyler AL, Wiekhorst F, Trahms L, Lindquist R, Schellenberger E, Mueller S, Schütz G, Roohi F, Ide A, Füchtemeier M, Gertz K, Kronenberg G, Harms U, Endres M, Dirnagl U, Farr TD (2013) Certain types of iron oxide nanoparticles are not suited to passively target inflammatory cells that infiltrate the brain in response to stroke. J Cereb Blood Flow Metab 36(Suppl 1):139–140. https://doi.org/10.1038/jcbfm.2013.22
Ebner B, Behm P, Jacoby C, Burghoff S, French BA, Schrader J, Flögel U (2010) Early assessment of pulmonary inflammation by 19F MRI in vivo. Circ Cardiovasc Imaging 3:202–210. https://doi.org/10.1161/CIRCIMAGING.109.902312
Stoll G, Basse-Lüsebrink T, Weise G, Jakob P (2012) Visualization of inflammation using 19F-magnetic resonance imaging and perfluorocarbons. Wiley Interdiscip Rev Nanomedicine Nanobiotechnology 4:438–447. https://doi.org/10.1002/wnan.1168
Shin SH, Kadayakkara DK, Bulte JWM (2017) In Vivo 19 F MR imaging cell tracking of inflammatory macrophages and site-specific development of colitis-associated dysplasia. Radiology 282:194–201. https://doi.org/10.1148/radiol.2016152387
Jacoby C, Borg N, Heusch P, Sauter M, Bönner F, Kandolf R, Klingel K, Schrader J, Flögel U (2014) Visualization of immune cell infiltration in experimental viral myocarditis by 19F MRI in vivo. Magn Reson Mater Phy. https://doi.org/10.1007/s10334-013-0391-6
Tirotta I, Dichiarante V, Pigliacelli C, Cavallo G, Terraneo G, Bombelli FB, Metrangolo P, Resnati G (2015) 19F magnetic resonance imaging (MRI): from design of materials to clinical applications. Chem Rev 115:1106–1129
Janjic JM, Ahrens ET (2009) Fluorine-containing nanoemulsions for MRI cell tracking. Wiley Interdiscip Rev Nanomedicine Nanobiotechnology 1:492–501
Westermann J, Söllner S, Ehlers E-M, Nohroudi K, Blessenohl M, Kalies K (2003) Analyzing the migration of labeled T cells in vivo: an essential approach with challenging features. Lab Investig 83:459–469. https://doi.org/10.1097/01.LAB.0000062852.80567.90
Gonzales C, Yoshihara HAI, Dilek N, Leignadier J, Irving M, Mieville P, Helm L, Michielin O, Schwitter J (2016) In-vivo detection and tracking of T cells in various organs in a melanomatumor model by 19F-fluorine MRS/MRI. PLoS One 11:1–18. https://doi.org/10.1371/journal.pone.0164557
Srinivas M, Morel PA, Ernst LA, Laidlaw DH, Ahrens ET (2007) Fluorine-19 MRI for visualization and quantification of cell migration in a diabetes model. Magn Reson Med 58:725–734. https://doi.org/10.1002/mrm.21352
Przybylski S, Gasch M, Marschner A, Ebert M, Ewe A, Helmig G, Hilger N, Fricke S, Rudzok S, Aigner A, Burkhardt J (2017) Influence of nanoparticle-mediated transfection on proliferation of primary immune cells in vitro and in vivo. PLoS One 12:1–16. https://doi.org/10.1371/journal.pone.0176517
Wayteck L, Dewitte H, De Backer L, Breckpot K, Demeester J, De Smedt SC, Raemdonck K (2016) Hitchhiking nanoparticles: reversible coupling of lipid-based nanoparticles to cytotoxic T lymphocytes. Biomaterials 77:243–254. https://doi.org/10.1016/j.biomaterials.2015.11.016
Liang S, Louchami K, Holvoet B, Verbeke R, Deroose CM, Manshian B, Soenen SJ, Lentacker I, Himmelreich U (2018) Tri-modal in vivo imaging of pancreatic islets transplanted subcutaneously in mice. Mol Imaging Biol 20:940–951. https://doi.org/10.1007/s11307-018-1192-0
Ferreira GB, Gysemans CA, Demengeot J, da Cunha JPMCM, Vanherwegen A-S, Overbergh L, Van Belle TL, Pauwels F, Verstuyf A, Korf H, Mathieu C (2014) 1,25-Dihydroxyvitamin D3 promotes tolerogenic dendritic cells with functional migratory properties in NOD mice. J Immunol 192:4210–4220. https://doi.org/10.4049/jimmunol.1302350
Poulin M, Haskins K (2000) Induction of diabetes in nonobese diabetic mice by Th2 T cell clones from a TCR transgenic mouse. J Immunol 164:3072–3078
Eizirik DL, Colli ML, Ortis F (2009) The role of inflammation in insulitis and beta-cell loss in type 1 diabetes. Nat Rev Endocrinol 5:219–226. https://doi.org/10.1038/nrendo.2009.21
Ahrens ET, Bulte JWM (2013) Tracking immune cells in vivo using magnetic resonance imaging. Nat Rev Immunol 13:755–763
van Heeswijk RB, Pellegrin M, Flögel U, Gonzales C, Aubert J-F, Mazzolai L, Schwitter J, Stuber M (2015) Fluorine MR imaging of inflammation in atherosclerotic plaque in vivo. Radiology 275:421–429. https://doi.org/10.1148/radiol.14141371
Temme S, Bönner F, Schrader J, Flögel U (2012) 19F magnetic resonance imaging of endogenous macrophages in inflammation. Wiley Interdiscip Rev Nanomedicine Nanobiotechnology 4:329–343. https://doi.org/10.1002/wnan.1163
Gaglia JL, Guimaraes AR, Harisinghani M, Turvey SE, Jackson R, Benoist C, Mathis D, Weissleder R (2011) Noninvasive imaging of pancreatic islet inflammation in type 1A diabetes patients. J Clin Invest 121:442–445. https://doi.org/10.1172/JCI44339
Chapelin F, Gao S, Okada H, Weber TG, Messer K, Ahrens ET (2017) Fluorine-19 nuclear magnetic resonance of chimeric antigen receptor T cell biodistribution in murine cancer model. Sci Rep 7:1–5. https://doi.org/10.1038/s41598-017-17669-4
O’Hanlon CF, Fedczyna T, Eaker S, Shingleton WD, Helfer BM (2017) Integrating a 19F MRI tracer agent into the clinical scale manufacturing of a T-cell immunotherapy. Contrast Media Mol Imaging 201:1–7. https://doi.org/10.1155/2017/9548478
Amiri H, Srinivas M, Veltien A, van Uden MJ, de Vries IJM, Heerschap A (2015) Cell tracking using 19F magnetic resonance imaging: technical aspects and challenges towards clinical applications. Eur Radiol 25:726–735. https://doi.org/10.1007/s00330-014-3474-5
Boehm-Sturm P, Mengler L, Wecker S, Hoehn M, Kallur T (2011) In Vivo tracking of human neural stem cells with 19F magnetic resonance imaging. PLoS One 6:e29040. https://doi.org/10.1371/journal.pone.0029040
Waiczies S, Millward JM, Starke L, Delgado PR, Huelnhagen T, Prinz C, Marek D, Di Wecker, Wissmann R, Koch SP, Boehm-Sturm P, Waiczies H, Niendorf T, Pohlmann A (2017) Enhanced fluorine-19 MRI sensitivity using a cryogenic radiofrequency probe: technical developments and ex vivo demonstration in a mouse model of neuroinflammation. Sci Rep 7:1–10. https://doi.org/10.1038/s41598-017-09622-2
Liang S, Dresselaers T, Louchami K, Zhu C, Liu Y, Himmelreich U (2017) Comparison of different compressed sensing algorithms for low SNR 19F MRI applications—imaging of transplanted pancreatic islets and cells labeled with perfluorocarbons. NMR Biomed 30:e3776. https://doi.org/10.1002/nbm.3776
Acknowledgements
The authors are grateful for financial support by the European Commission for the FP7 MC-ITN ‘BetaTrain’ (EU-FP7/207-2013/ 289932), by the European ERA-NET project ‘CryptoView’ (3rd call of the FP7 programme Infect-ERA), by the Flemish Wetenschap Onderzoek (FWO) for the projects G.0B28.14 and G.0A75.14, by the Agentschap Innoveren & Ondernemen for the IWT-SBO ‘NanoComit’ (140061).
Author information
Authors and Affiliations
Contributions
Study conception and design: SS, HK, IL, UH. Methodology: SL, HK, RV, KR, CG. Experimentation: SS, HK, BM, RV, SL. Analysis and interpretation of data: SS, HK, BM, UH. Drafting of manuscript: SS. Critical revision: HK, CG, IL, SD, UH.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants performed by any of the authors. Experiments involving mice were performed in accordance with regional, national and international standards on animal welfare, in particular the European Union Directive 2010/63/EU, and approved and overseen by the Animal Care and Ethical Committees of the University of Leuven.
Rights and permissions
About this article
Cite this article
Saini, S., Korf, H., Liang, S. et al. Challenges for labeling and longitudinal tracking of adoptively transferred autoreactive T lymphocytes in an experimental type-1 diabetes model. Magn Reson Mater Phy 32, 295–305 (2019). https://doi.org/10.1007/s10334-018-0720-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10334-018-0720-x