Abstract
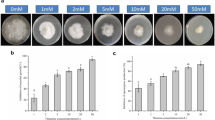
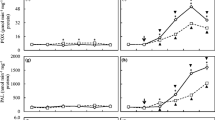
In leaves pretreated with an indole derivative [indole-3-acetic acid (IAA) tryptamine, or tryptophan], blast lesion formation was suppressed compared to those treated with distilled water (DW) as a control. Phenylalanine ammonia-lyase (PAL) activity and PAL expression were significantly enhanced in the IAA- or tryptophan-pretreated leaves, but not in tryptamine-pretreated leaves. This induction of resistance was inhibited by pretreatment with a PAL inhibitor, α-aminooxyacetic acid, in IAA- and in tryptophan-treated leaves, but not in tryptamine-treated leaves. This study strongly shows that the indole derivatives IAA tryptamine and tryptophan can enhance a disease resistance mechanism that is supported by different metabolic pathways.




Similar content being viewed by others
References
Arase S, Fujita K, Uehara T, Honda Y, Isota J (2000) Light-enhanced resistance to Magnaporthe grisea infection in the rice Sekiguchi lesion mutants. J Phytopathol 148:197–203
Arase S, Ueno M, Toko M, Honda Y, Itho K, Ozoe Y (2001) Light-dependent accumulation of tryptamine in the rice Sekiguchi lesion mutant infected with Magnaporthe grisea. J Phytopathol 149:409–413
Dixon RA, Lamb CJ (1990) Molecular communication in interactions between plants and microbial pathogens. Annu Rev Plant Physiol Plant Mol Biol 41:339–367
Erdmann N, Schiewer U (1971) Tryptophan-dependent indoleacetic acid biosynthesis from indole, demonstrated by double-labeling experiments. Planta 97:135–141
Fujita K, Arase S, Hiratsuka H, Honda Y, Nozu M (1994) The role of toxin(s) produced by germinating spores of Pyricularia oryzae in pathogenesis. J Phytopathol 142:245–252
Hahlbrock K, Scheel D (1989) Physiology and molecular biology of phenylpropanoid metabolism. Annu Rev Plant Physiol Plant Mol Biol 40:347–369
Iwata M, Sekizawa Y, Iwamatsu H, Suzuki Y, Watanabe T (1981) Effects of plant hormones on peroxidase activity in rice leaf and incidence of rice blast. Ann Phytopathol Soc Jpn 47:646–653
Matsuo H, Taniguchi K, Hiramoto T, Yamada T, Ichinose Y, Toyoda K, Takeda K, Shiraishi T (2001) Gramine increase associated with rapid and transient systemic resistance in barley induced by mechanical and biological stresses. Plant Cell Physiol 42:1103–1111
Miyagawa H, Toda H, Tsurushima T, Ueno T, Shishiyama J (1994) Accumulation of tryptamine in barley leaves irradiated with UV light. Biosci Biotechnol Biochem 58:1723–1724
Silva MC, Nicole M, Guerra-Guimarães L, Rodrigues CJ Jr (2002) Hypersensitive cell death and post-haustorial defence responses arrest the orange rust (Hemileia vastatrix) growth in resistant coffee leaves. Physiol Mol Plant Pathol 60:169–183
Sticher L, Mauch-Mani B, Metraux JP (1997) Systemic acquired resistance. Annu Rev Phytopathol 35:235–270
Thomas JC, Adams DG, Nessler CL, Brown JK, Bohnert HJ (1995) Tryptophan decarboxylase, tryptamine, and reproduction of the whitefly. Plant Physiol 109:717–720
Tomiyama K (1963) Physiology and biochemistry of disease resistance of plants. Annu Rev Phytopathol 1:295–324
Ueno M, Shibata H, Kihara J, Honda Y, Arase S (2003) Increased tryptophan decarboxylase and monoamine oxidase activities induce Sekiguchi lesion formation in rice infected with Magnaporthe grisea. Plant J 36:215–228
Verwoerd TC, Dekker BMM, Hoekema A (1989) A small-scale procedure for the rapid isolation of plant RNAs. Nucleic Acids Res 17:2362
Watanabe T, Sekizawa Y, Shimura M, Suzuki Y, Matsumoto K, Iwata M, Mase S (1979) Rice blast controlling agent of benzisothiazole analogs III. Effects of probenazole (Oryzaemate®) on rice plants with reference to controlling rice blast. J Pestic Sci 4:53–59
Acknowledgments
This study was supported in part by a Grant-in-Aid for Young Scientists (B) (No. 21780037) from the Ministry of Education, Sports, Science and Technology of Japan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ueno, M., Kumura, Y., Ueda, K. et al. Indole derivatives enhance resistance against the rice blast fungus Magnaporthe oryzae . J Gen Plant Pathol 77, 209–213 (2011). https://doi.org/10.1007/s10327-011-0300-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10327-011-0300-7