Abstract
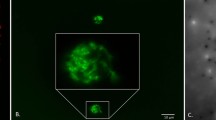
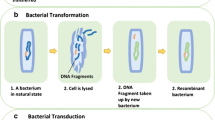
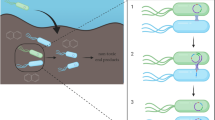
Horizontal gene transfer (HGT) is the lateral movement of genetic material between organisms. The RDX explosive-degrading bacterium Gordonia sp. KTR9 has been shown previously to transfer the pGKT2 plasmid containing the RDX degradative genes (xplAB) by HGT. Overall, fitness costs to the transconjugants to maintain pGKT2 was determined through growth and survivability assessments. Rhodococcus jostii RHA1 transconjugants demonstrated a fitness cost while other strains showed minimal cost. Biogeochemical parameters that stimulate HGT of pGKT2 were evaluated in soil slurry mating experiments and the absence of nitrogen was found to increase HGT events three orders of magnitude. Experiments evaluating RDX degradation in flow-through soil columns containing mating pairs showed 20% greater degradation than columns with only the donor KTR9 strain. Understanding the factors governing HGT will benefit bioaugmentation efforts where beneficial bacteria with transferrable traits could be used to more efficiently degrade contaminants through gene transfer to native populations.





Similar content being viewed by others
References
Aspray TA, Hansen SK, Burns RG (2005) A soil-based microbial biofilm exposed to 2,4-D: bacterial community development and establishment of conjugative plasmid pJP4. FEMS Microbiol Ecol 54:317–327
Baltrus D (2013) Exploring the costs of horizontal gene transfer. Trends Ecol Evol 28:489–495
Beane NR, VanZomeran CM, Price CL, Seiter-Moser JM, Conkle JL, Stevens BN, Berkowitz JF (eds) (2017) Integrated assessment of vegetation and soil conditions following herbicide application. In: ERDC Technical Report, vol ERDC/EL TR-17-9. Engineer Research and Development Center
Brophy JAN, Triassi AJ, Adams BL, Renberg RL, Stratis-Cullum DN, Grossman AD, Voigt CA (2018) Engineered integrative and conjugative elements for efficient and inducible DNA transfer to undomesticated bacteria. Nat Microbiol 3:1043–1053
Carroll AC, Wong A (2018) Plasmid persistence: costs, benefits, and the plasmid paradox. Can J Microbiol 64:293–304
Christensen BB, Sternberg C, Andersen JB, Eberl L, Moller S, Givskov M, Molin S (1998) Establishment of new genetic traits in a microbial biofilm community. Appl Environ Microbiol 64:2247–2255
Crocker F, Indest KJ, Jung CM, Hancock DE, Merritt MM, Florizone C, Chen H-P, Stewart GR, Zhu S, Sukdeo N, Fortin M-C, Hallam SJ, Mohn WW, Eltis LD, Perreault NN, Zhao J-S, Paquet L, Halasz A, Hawari J (eds) (2012) Identification of microbial gene biomarkers for in situ RDX biodegradation. In: Strategic Environmental Research and Development Program; Project ER-1609. ERDC Technical Report, vol ERDC/EL TR-12-33
Crocker FH, Indest KJ, Jung CM, Hancock DE, Fuller ME, Hatzinger PB, Vainberg S, Istok JD, Wilson E, Michalsen MM (2015) Evaluation of microbial transport during aerobic bioaugmentation of an RDX-contaminated aquifer. Biodegradation 26:443–451
Crocker FH, Thompson KT, Szecsody JE, Fredrickson HL (2005) Biotic and abiotic degradation of CL-20 and RDX in soils. J Environ Qual 34:2208–2216
De Gelder L, Vandecasteele FPJ, Brown CJ, Forney LJ, Top EM (2005) Plasmid donor affects host range of promiscuous IncP-1b plasmid pB10 in an activated-sludge microbial community. Appl Environ Microbiol 71:5309–5317
Dejonghe W, Goris J, El Fantroussi S, Hofte M, De Vos P, Verstraete W, Top EM (2000) Effect of dissemination of 2,4-dichlorophenoxyacetic acid (2,4-D) degradation plasmids on 2,4-d degradation and on bacterial community structure in two different soil horizons. Appl Environ Microbiol 66:3297–3304
Dionisio F, Conceicao I, Marques A, Fernandes L, Gordo I (2005) The evolution of a conjugative plasmid and its ability to increase bacterial fitness. Biol Lett 1:250–252
Ellis DE, Lutz EJ, Odom JM, Buchanan RJ, Bartlett CL, Lee MD, Harkness MR, DeWeerd KA (2000) Bioaugmentation for accelerated in situ anaerobic bioremediation. Environ Sci Technol 34:2254–2260
Fuller M, Hatzinger P, Condee C, Andaya C, Michalsen M, Crocker F, Indest K, Jung C, Hancock D, Istok J (2015) Laboratory evaluation of bioaugmentation for aerobic treatment of RDX in groundwater. Biodegradation 2:77–89
Fuller ME, Hatzinger PB, Condee CW, Andaya C, Vainberg S, Michalsen MM, Crocker FH, Indest KJ, Jung CM, Eaton H, Istok JD (2015) Laboratory evaluation of bioaugmentation for aerobic treatment of RDX in groundwater. Biodegradation 26:77–89
Garbisu C, Garaiyurrebaso O, Epelde L, Grohmann E, Alkorta I (2017) Plasmid-mediated bioaugmentation for the bioremediation of contaminated soils. Front Microbiol 8:1966
Ikuma K, Gunsch C (2012) Genetic bioaugmentation as an effective method for in situ bioremediation: functionality of catabolic plasmids following conjugal transfers. Bioengineered 3:234–241
Ikuma K, Holzem R, Gunsch C (2012) Impacts of organic carbon availability and recipient bacteria characteristics on the potential for TOL plasmid genetic bioaugmentation in soil slurries. Chemosphere 89:158–163
Indest KJ, Hancock D, Jung CM, Eberly JO, Mohn WW, Eltis LD, Crocker FH (2013) Role of nitrogen limitation in transformation of RDX (hexahydro-1,3,5-trinitro-1,3,5-triazine) by Gordonia sp. strain KTR9. Appl Environ Microbiol 79:1746–1750
Indest KJ, Jung CM, Chen HP, Hancock D, Florizone C, Eltis LD, Crocker FH (2010) Functional characterization of pGKT2, a 182 kb plasmid containing the xplAB genes involved in the degradation of RDX by Gordonia sp. KTR9. Appl Environ Microbiol 76:6329–6337
Jung CM, Crocker FH, Eberly JO, Indest KJ (2011) Horizontal gene transfer (HGT) as a mechanism of dissemination of RDX-degrading activity among Actinomycete bacteria. J Appl Microbiol 110:1449–1459
Lendvay JM, Löffler FE, Dollhopf M, Aiello MR, Daniels G, Fathepure BZ, Gebhard M, Heine R, Helton R, Shi J, Krajmalnik-Brown R, Major CL, Barcelona MJ, Petrovskis E, Hickey R, Tiedje JM, Adriaens P (2003) Bioreactive barriers: a comparison of bioaugmentation and biostimulation for chlorinated solvent remediation. Environ Sci Technol 37:1422–1431
Michalsen MM, King AS, Rule RA, Fuller ME, Hatzinger PB, Condee CW, Crocker FH, Indest KJ, Jung CM, Istok JD (2016) Evaluation of biostimulation and bioaugmentation to stimulate hexahydro-1,3,5-trinitro-1,3,5,-triazine degradation in an aerobic groundwater aquifer. Environ Sci Technol 50:7625–7632
Mølbeck L, Søren M, Kroer N (2007) Root growth and exudate production define the frequency of horizontal plasmid transfer in the rhizosphere. FEMS Microbiol Ecol 59:167–176
Musovic S, Dechesne A, Sorensen J, Smets BF (2010) A novel assay to assess permissiveness of a soil microbial community toward receipt of mobile genetic elements. Appl Environ Microbiol 76:4813–4818
Nielsen KM, Bøhn T, Townsend JP (2014) Detecting rare transfer events in bacterial populations. Front Microbiol 4:1–12
Pope C, McHugh T, Gillespie S (2010) Methods to determine fitness in bacteria. In: McHugh SGaT (ed) Methods in molecular biology, vol 642, antibiotic resistance protocols, 2nd edn. Springer Science, Berlin, pp 113–121
Ren C, Wang Y, Tian L, Chen M, Sun J, Li L (2018) Genetic bioaugmentation of activated sludge with dioxin-catabolic plasmids harbored by Rhodococcus sp. strain p52. Environ Sci Technol 52:5339–5348
Rösch T, Golman W, Hucklesby L, Gonzalez-Pastor J, Graumann P (2014) The presence of conjugative plasmid pLS20 affects global transcription of its Bacillis subtilis host and confers beneficial stress resistance to cells. Appl Environ Microbiol 80:1349–1358
Rylott E, Budarina MV, Barker A, Lorenz A, Strand S, Bruce CN (2011) Engineering plants for the phytoremediation of RDX in the presence of the co-contaminating explosive TNT. New Phytol 192:405–413
Rylott EL, Bruce NC (2008) Plants disarm soil: engineering plants for the phytoremediation of explosives. Trends Biotechnol 27:73–81
San Millan A, Maclean RC (2017) Fitness costs of plasmids: a limit to plasmid transmission. In: Microbiol Spectrum 5:MTBP-0016-2017
Seoane J, Yankelvich T, Dechesne A, Merkey B, Sternberg C, Smets BF (2011) An individual-based approach to explain plasmid invasion in bacterial population. FEMS Microbiol Ecol 75:17–27
Shintani M, Takahashi Y, Tokumaru H, Kadota K, Hara H, Miyakoshi M, Naito K, Yamane H, Nishida H, Nojiri H (2010) Response of the Pseudomonas host chromosomal transcriptome to carriage of the IncP-7 plasmid pCAR1. Environ Microbiol 12:1413–1426
Sorensen SJ, Bailey M, Hansen LH, Kroer N, Wuertz S (2005) Studying plasmid horizontal transfer in situ: a critical review. Nat Rev Microbiol 3:700–710
Suja F, Rahim F, Taha MR, Hambali N, Rizal Razali M, Khalid A, Hamzah A (2014) Effects of local microbial bioaugmentation and biostimulation on the bioremediation of total petroleum hydrocarbons (TPH) in crude oil contaminated soil based on laboratory and field observations. Int Biodeter Biodegr 90:115–122
Sun J, Qiu Y, Ding P, Peng P, Yang H, Li L (2017) Conjugative transfer of dioxin–catabolic megaplasmids and bioaugmentation prospects of a Rhodococcus sp. Environ Sci Technol 51:6298–6307
Top EM, Springael D, Boon N (2002) Catabolic mobile genetic elements and their potential use in bioaugmentation of polluted soils and waters. FEMS Microbiol Ecol 42:199–208
Top EM, Van Daele P, De Saeyer N, Forney LJ (1998) Enhancement of 2,4-dichlorophenoxyacetic acid (2,4-D) degradation in soil by dissemination of catabolic plasmids. Antonie Van Leeuwenhoek 73:87–94
Wiedenbeck J, Cohan F (2011) Origins of bacterial diversity through horizontal genetic transfer and adaptation to new ecological niches. FEMS Microbiol Rev 35:957–976
Wu M, Dick WA, Li W, Wang X, Yang Q, Wang T, Xu L, Zhang M, Chen L (2016) Bioaugmentation and biostimulation of hydrocarbon degradation and the microbial community in a petroleum-contaminated soil. Int Biodeter Biodegr 107:158–164
Zhang L, Routsong R, Nguyen Q, Rylott EL, Bruce NC, Strand SE (2017) Expression in grasses of multiple transgenes for degradation of munitions compounds on live-fire training ranges. Plant Biotechnol J 15:624–633
Acknowledgements
This research was funded through the US Army Corps of Engineers’ Environmental Quality Program. Views, opinions and/or findings contained herein are those of the authors and should not be construed as an Official Department of the Army position or decision unless so designated by other official documentation. The authors would like to thank Mrs. Cynthia Price for the use of peristaltic pumps and columns and for her scientific advice.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Jung, C.M., Carr, M., Blakeney, G.A. et al. Enhanced plasmid-mediated bioaugmentation of RDX-contaminated matrices in column studies using donor strain Gordonia sp. KTR9. J Ind Microbiol Biotechnol 46, 1273–1281 (2019). https://doi.org/10.1007/s10295-019-02185-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-019-02185-3