Abstract
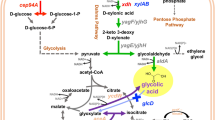
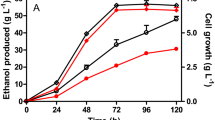
The non-conventional d-xylose metabolism called the Dahms pathway which only requires the expression of at least three enzymes to produce pyruvate and glycolaldehyde has been previously engineered in Escherichia coli. Strains that rely on this pathway exhibit lower growth rates which were initially attributed to the perturbed redox homeostasis as evidenced by the lower intracellular NADPH concentrations during exponential growth phase. NADPH-regenerating systems were then tested to restore the redox homeostasis. The membrane-bound pyridine nucleotide transhydrogenase, PntAB, was overexpressed and resulted to a significant increase in biomass and glycolic acid titer and yield. Furthermore, expression of PntAB in an optimized glycolic acid-producing strain improved the growth and product titer significantly. This work demonstrated that compensating for the NADPH demand can be achieved by overexpression of PntAB in E. coli strains assimilating d-xylose through the Dahms pathway. Consequently, increase in biomass accumulation and product concentration was also observed.






Similar content being viewed by others
References
Andersen KB, von Meyenburg K (1977) Charges of nicotinamide adenine nucleotides and adenylate energy charge as regulatory parameters of the metabolism in Escherichia coli. J Biol Chem 252:4151–4156
Balzer GJ, Thakker C, Bennett GN, San K-Y (2013) Metabolic engineering of Escherichia coli to minimize byproduct formate and improving succinate productivity through increasing NADH availability by heterologous expression of NAD+-dependent formate dehydrogenase. Metab Eng 20:1–8. https://doi.org/10.1016/j.ymben.2013.07.005
Bologna FP, Andreo CS, Drincovich MF (2007) Escherichia coli malic enzymes: two isoforms with substantial differences in kinetic properties, metabolic regulation, and structure. J Bacteriol 189:5937–5946. https://doi.org/10.1128/JB.00428-07
Cabulong RB, Lee W-K, Bañares AB et al (2018) Engineering Escherichia coli for glycolic acid production from d-xylose through the Dahms pathway and glyoxylate bypass. Appl Microbiol Biotechnol 102:2179–2189. https://doi.org/10.1007/s00253-018-8744-8
Cabulong RB, Valdehuesa KNG, Ramos KRM et al (2017) Enhanced yield of ethylene glycol production from d-xylose by pathway optimization in Escherichia coli. Enzym Microb Technol 97:11–20. https://doi.org/10.1016/j.enzmictec.2016.10.020
Choi SY, Park SJ, Kim WJ et al (2016) One-step fermentative production of poly(lactate-co-glycolate) from carbohydrates in Escherichia coli. Nat Biotechnol 34:435–440. https://doi.org/10.1038/nbt.3485
Cui Y-Y, Ling C, Zhang Y-Y et al (2014) Production of shikimic acid from Escherichia coli through chemically inducible chromosomal evolution and cofactor metabolic engineering. Microb Cell Fact 13:21. https://doi.org/10.1186/1475-2859-13-21
Dahms AS (1974) 3-Deoxy-d-pentulosonic acid aldolase and its role in a new pathway of d-xylose degradation. Biochem Biophys Res Comm 60:1433–1439. https://doi.org/10.1016/0006-291X(74)90358-1
Green MR, Sambrook J (2012) Molecular cloning: a laboratory manual, 4th edn. Cold Spring Harbor Laboratory Press, New York
Haverkorn van Rijsewijk BRB, Kochanowski K, Heinemann M, Sauer U (2016) Distinct transcriptional regulation of the two Escherichia coli transhydrogenases PntAB and UdhA. Microbiol 162:1672–1679. https://doi.org/10.1099/mic.0.000346
Holm AK, Blank LM, Oldiges M et al (2010) Metabolic and transcriptional response to cofactor perturbations in Escherichia coli. J Biol Chem 285:17498–17506. https://doi.org/10.1074/jbc.M109.095570
Khattab SMR, Saimura M, Kodaki T (2013) Boost in bioethanol production using recombinant Saccharomyces cerevisiae with mutated strictly NADPH-dependent xylose reductase and NADP+-dependent xylitol dehydrogenase. J Biotechnol 165:153–156. https://doi.org/10.1016/j.jbiotec.2013.03.009
Lee W-H, Kim M-D, Jin Y-S, Seo J-H (2013) Engineering of NADPH regenerators in Escherichia coli for enhanced biotransformation. Appl Microbiol Biotechnol 97:2761–2772. https://doi.org/10.1007/s00253-013-4750-z
Lim JH, Seo SW, Kim SY, Jung GY (2013) Model-driven rebalancing of the intracellular redox state for optimization of a heterologous n-butanol pathway in Escherichia coli. Metab Eng 20:56–62. https://doi.org/10.1016/j.ymben.2013.09.003
Lipson DA (2015) The complex relationship between microbial growth rate and yield and its implications for ecosystem processes. Front Microbiol 6:615. https://doi.org/10.3389/fmicb.2015.00615
Liu H, Ramos KRM, Valdehuesa KNG et al (2013) Biosynthesis of ethylene glycol in Escherichia coli. Appl Microbiol Biotechnol 97:3409–3417. https://doi.org/10.1007/s00253-012-4618-7
Liu J, Li H, Zhao G et al (2018) Redox cofactor engineering in industrial microorganisms: strategies, recent applications and future directions. J Ind Microbiol Biotechnol 45:313–327. https://doi.org/10.1007/s10295-018-2031-7
Meijnen J-P, de Winde JH, Ruijssenaars HJ (2009) Establishment of oxidative d-xylose metabolism in Pseudomonas putida S12. Appl Environ Microbiol 75:2784–2791. https://doi.org/10.1128/AEM.02713-08
Radek A, Krumbach K, Gätgens J et al (2014) Engineering of Corynebacterium glutamicum for minimized carbon loss during utilization of d-xylose containing substrates. J Biotechnol 192(Pt A):156–160. https://doi.org/10.1016/j.jbiotec.2014.09.026
Rathnasingh C, Raj SM, Lee Y et al (2012) Production of 3-hydroxypropionic acid via malonyl-CoA pathway using recombinant Escherichia coli strains. J Biotechnol 157:633–640. https://doi.org/10.1016/j.jbiotec.2011.06.008
Salusjärvi L, Toivari M, Vehkomäki M-L et al (2017) Production of ethylene glycol or glycolic acid from d-xylose in Saccharomyces cerevisiae. Appl Microbiol Biotechnol 101:8151–8163. https://doi.org/10.1007/s00253-017-8547-3
Sauer U, Canonaco F, Heri S et al (2004) The soluble and membrane-bound transhydrogenases UdhA and PntAB have divergent functions in NADPH metabolism of Escherichia coli. J Biol Chem 279:6613–6619. https://doi.org/10.1074/jbc.M311657200
Shi A, Zhu X, Lu J et al (2013) Activating transhydrogenase and NAD kinase in combination for improving isobutanol production. Metab Eng 16:1–10. https://doi.org/10.1016/j.ymben.2012.11.008
Spaans SK, Weusthuis RA, van der Oost J, Kengen SWM (2015) NADPH-generating systems in bacteria and archaea. Front Microbiol 6:159. https://doi.org/10.3389/fmicb.2015.00742
Stockland AE, San Clemente CL (1969) Multiple forms of lactate dehydrogenase in Staphylococcus aureus. J Bacteriol 100:347–353
Valdehuesa KNG, Lee W-K, Ramos KRM et al (2015) Identification of aldehyde reductase catalyzing the terminal step for conversion of xylose to butanetriol in engineered Escherichia coli. Bioproc Biosyst Eng 38:1761–1772. https://doi.org/10.1007/s00449-015-1417-4
Valdehuesa KNG, Liu H, Ramos KRM et al (2014) Direct bioconversion of d-xylose to 1,2,4-butanetriol in an engineered Escherichia coli. Proc Biochem 49:25–32. https://doi.org/10.1016/j.procbio.2013.10.002
Valdehuesa KNG, Ramos KRM, Nisola GM et al (2018) Everyone loves an underdog: metabolic engineering of the xylose oxidative pathway in recombinant microorganisms. Appl Microbiol Biotechnol 102:7703–7716. https://doi.org/10.1007/s00253-018-9186-z
Wang Y, San K-Y, Bennett GN (2013) Cofactor engineering for advancing chemical biotechnology. Curr Opin Biotechnol 24:994–999. https://doi.org/10.1016/j.copbio.2013.03.022
Wang Y, San K-Y, Bennett GN (2013) Improvement of NADPH bioavailability in Escherichia coli by replacing NAD+-dependent glyceraldehyde-3-phosphate dehydrogenase GapA with NADP+-dependent GapB from Bacillus subtilis and addition of NAD kinase. J Ind Microbiol Biotechnol 40:1449–1460. https://doi.org/10.1007/s10295-013-1335-x
Wasserstrom L, Portugal-Nunes D, Almqvist H et al (2018) Exploring d-xylose oxidation in Saccharomyces cerevisiae through the Weimberg pathway. AMB Express 8:33. https://doi.org/10.1186/s13568-018-0564-9
Weimberg R (1961) Pentose oxidation by Pseudomonas fragi. J Biol Chem 236:629–635
Xu P, Vansiri A, Bhan N, Koffas MAG (2012) ePathBrick: a synthetic biology platform for engineering metabolic pathways in E. coli. ACS Synth Biol 1:256–266. https://doi.org/10.1021/sb300016b
Zhao C, Zhao Q, Li Y, Zhang Y (2017) Engineering redox homeostasis to develop efficient alcohol-producing microbial cell factories. Microb Cell Fact 16:115. https://doi.org/10.1186/s12934-017-0728-3
Zhao H, van der Donk WA (2003) Regeneration of cofactors for use in biocatalysis. Curr Opin Biotechnol 14:583–589. https://doi.org/10.1016/j.copbio.2003.09.007
Funding
This work was supported by Korea Research Fellowship Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science and ICT (2015H1D3A1062172 and 2016R1C1B1013252) and by the Ministry of Education (No. 2018R1D1A1B07043993 and No. 2009-0093816).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants performed by any of the authors.
Rights and permissions
About this article
Cite this article
Cabulong, R.B., Valdehuesa, K.N.G., Bañares, A.B. et al. Improved cell growth and biosynthesis of glycolic acid by overexpression of membrane-bound pyridine nucleotide transhydrogenase. J Ind Microbiol Biotechnol 46, 159–169 (2019). https://doi.org/10.1007/s10295-018-2117-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-018-2117-2