Abstract
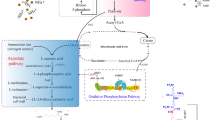
l-Lysine is widely used as a nutrition supplement in feed, food, and beverage industries as well as a chemical intermediate. At present, great efforts are made to further decrease the cost of lysine to make it more competitive in the markets. Furthermore, lysine also shows potential as a feedstock to produce other high-value chemicals for active pharmaceutical ingredients, drugs, or materials. In this review, the current biomanufacturing of lysine is first presented. Second, the production of novel derivatives from lysine is discussed. Some chemicals like l-pipecolic acid, cadaverine, and 5-aminovalerate already have been obtained at a lab scale. Others like 6-aminocaproic acid, valerolactam, and caprolactam could be produced through a biological and chemical coupling pathway or be synthesized by a hypothetical pathway. This review demonstrates an active and expansive lysine industry, and these green biomanufacturing strategies could also be applied to enhance the competitiveness of other amino acid industry.








Similar content being viewed by others
Abbreviations
- Lys:
-
Lysine
- l-PA:
-
l-Pipecolic acid
- LCD:
-
Lysine cyclodeaminase
- LDC:
-
l-Lysine decarboxylase
- GDH:
-
Glucose dehydrogenase
- LysR:
-
Lysine racemase
- 6ACA:
-
6-Aminocaproic acid
- 5AVA:
-
5-Aminovalerate
References
Adger B, Dyer U, Hutton G, Woods M (1996) Stereospecific synthesis of the anaesthetic levobupivacaine. Tetrahedron Lett 37:6399–6402. https://doi.org/10.1016/0040-4039(96)01357-3
Ajinomoto Co. I (2014) First quarter-FY2014 Market and other information. Ajinomoto Co., Inc., Tokyo
Becker J, Wittmann C (2012) Bio-based production of chemicals, materials and fuels-Corynebacterium glutamicum as versatile cell factory. Curr Opin Biotechnol 23:631–640. https://doi.org/10.1016/j.copbio.2011.11.012
Becker J, Zelder O, Hafner S, Schröder H, Wittmann C (2011) From zero to hero-design-based system metabolic engineering of Corynebacterium glutamicum for l-lysine production. Metab Eng 13:159–168. https://doi.org/10.1016/j.ymben.2011.01.003
Binder S, Schendzielorz G, Stäbler N, Krumbach K, Hoffmann K, Bott M, Eggeling L (2012) A high-throughput approach to identify genomic variants of bacterial metabolite producers at the single-cell level. Genome Biol 13:R40. https://doi.org/10.1186/gb-2012-13-5-r40
Binder S, Siedler S, Marienhagen J, Bott M, Eggeling L (2013) Recombineering in Corynebacterium glutamicum combined with optical nanosensors: a general strategy for fast producer strain generation. Microb Biotechnol 6:131–140. https://doi.org/10.1093/nar/gkt312
Byun SM, Jeong SW, Cho DH, Kim YH (2015) Optimized conversion of l-lysine to l-pipecolic acid using recombinant lysine cyclodeaminase from Streptomyces pristinaespiralis. Biotechnol Bioprocess Eng 20:73–78. https://doi.org/10.1007/s12257-014-0428-3
Chae TU, Ko Y-S, Hwang K-S, Lee SY (2017) Metabolic engineering of Escherichia coli for the production of four-, five- and six-carbon lactams. Metab Eng 41:82–91. https://doi.org/10.1016/j.ymben.2017.04.001
Chattopadhyay SK, Biswas T, Biswas T (2008) Complementary routes to both enantiomers of pipecolic acid and 4,5-dihydroxypipecolic acid derivatives. Tetrahedron Lett 49:1365–1369. https://doi.org/10.1016/j.tetlet.2007.12.097
Contador C, Rizk M, Asenjo JA, Liao JC (2009) Ensemble modeling for strain development of l-lysine-producing Escherichia coli. Metab Eng 11:221–233. https://doi.org/10.1016/j.ymben.2009.04.002
Eggeling L, Bott M (2015) A giant market and a powerful metabolism: l-lysine provided by Coryne-bacterium glutamicum. Appl Microbiol Biot 99:3387–3394. https://doi.org/10.1007/s00253-015-6508-2
Eichhorn E, Roduit J, Shaw N, Heinzmann K, Kiener A (1997) Preparation of (S)-piperazine-2-carboxylic acid, (R)-piperazine-2-carboxylic acid, and (S)-piperidine-2-carboxylic acid by kinetic resolution of the corresponding racemic carboxamides with stereoselective amidases in whole bacterial cells. Tetrahedron Asymmetr 8:2533–2536. https://doi.org/10.1016/S0957-4166(97)00256-5
Faurie R, Thommel J, Bathe B, Debabov VG, Huebner S, Ikeda M, Kimura E, Marx A, Möckel B, Mueller U, Pfefferle W (2003) Microbial production of l-amino acids. Ikeda M (ed) Amino acid production process. Advances in biochemical engineering/biotechnology. Springer Verlag, Tokyo, pp 1–35
Frost JW (2005) Synthesis of caprolactam from lysine. WO123669 A1
Gao H, Zhuo Y, Ashforth E, Zhang LX (2010) Engineering of a genome-reduced host: practical application of synthetic biology in the overproduction of desired secondary metabolites. Protein Cell 1:621–626. https://doi.org/10.1007/s13238-010-0073-3
Gatto GJ, Boyne MT, Kelleher NL, Walsh CT (2006) Biosynthesis of pipecolic acid by RapL, a lysine cyclodeaminase encoded in the rapamycin gene cluster. J Am Chem Soc 128:3838–3847. https://doi.org/10.1021/ja0587603
Ghandi A, Powell IB, Broome M, Adhikari B (2013) Survival, fermentation activity and storage stability of spray dried Lactococcus lactis produced via different atomization regimes. J Food Eng 115:83–90. https://doi.org/10.1016/j.jfoodeng.2012.09.022
Gibbs FB, Kermasha S, Alli I, Mulligan CN (1999) Encapsulation in the food industry: a review. Int J Food Sci Nutr 50:213–224. https://doi.org/10.1080/096374899101256
Ginesta X, Pericas MA, Riera A (2002) Straightforward entry to the pipecolic acid nucleus. Enantioselective synthesis of baikiain. Tetrahedron Lett 43:779–782. https://doi.org/10.1016/S0040-4039(01)02271-7
Gopinath V, Meiswinkel TM, Wendisch VF, Nampoothiri M (2011) Amino acid production from rice straw and wheat bran hydrolysate by recombinant pentose-utilizing Corynebacterium glutamicum. Appl Microbiol Biotechnol 92:985–996. https://doi.org/10.1007/s00253-011-3478-x
Hajnal I (2016) Meeting report: cold spring harbor Asia synthetic biology meeting. Biotechnol J 11:197–198. https://doi.org/10.1002/biot.201400836
He M (2006) Pipecolic acid in microbes: biosynthetic routes and enzymes. J Ind Microbiol Biotechnol 33:401–407. https://doi.org/10.1007/s10295-006-0078-3
Heider SAE, Wendisch VF (2015) Engineering microbial cell factories: metabolic engineering of Corynebacterium glutamicum with a focus on non-natural products. Biotechnol J 10:1170–1184. https://doi.org/10.1002/biot.201400590
Henke NA, Wiebe D, Pérez-García F, Peters-Wendisch P, Wendisch VF (2018) Coproduction of cell-bound and secreted value-added compounds: simultaneous production of carotenoids and amino acids by Corynebacterium glutamicum. Bioresour Technol 247:744–752. https://doi.org/10.1016/j.biortech.2017.09.167
Horaczek A, Viernstein H (2004) Comparison of three commonly used drying technologies with respect to activity and longevity of aerial conidia of Beauveria brongniartii and Metarhizium anisopliae. Biol Control 31:65–71. https://doi.org/10.1016/j.biocontrol.2004.04.016
Howell DM, Harich K, Xu HM, White RH (2000) Identification of enzymes homologous to isocitrate dehydrogenase that are involved in coenzyme B and leucine biosynthesis in methanoarchaea. J Bacteriol 182:5013–5016. https://doi.org/10.1128/JB.182.17.5013-5016.2000
Ikeda M, Mizuno Y, Awane S, Hayashi M, Mitsuhashi S, Takeno S (2011) Identification and application of a different glucose uptake system that functions as an alternative to the phosphotransferase system in Corynebacterium glutamicum. Appl Microbiol Biotechnol 90:1443–1451. https://doi.org/10.1007/s00253-011-3210-x
Ikeda M, Noguchi N, Ohshita M, Senoo A, Mitsuhashi S, Takeno S (2015) A third glucose uptake bypass in Corynebacterium glutamicum ATCC 31833. Appl Microbiol Biotechnol 99:2741–2750. https://doi.org/10.1007/s00253-014-6323-1
Ikeda M (2016) Lysine fermentation: History and genome breeding. In: Scheper Th, Belkin S, Bley Th, Bohlmann J, Gu MB, Hu WS, Mattiasson B, Nielsen J, Seitz H, Ulber R, Zeng AP, Zhong JJ, Zhou W (eds) Advances in biochemical engineering/biotechnology. Springer, Berlin Heidelberg, pp 73–102
Imao K, Konishi R, Kishida M, Hirata Y, Segawa S, Adachi N, Matsuura R, Tsuge Y, Matsumoto T, Tanaka T, Kondo A (2017) 1,5-Diaminopentane production from xylooligosaccharides using metabolically engineered Corynebacterium glutamicum displaying beta-xylosidase on the cell surface. Bioresour Technol 245:1684–1691. https://doi.org/10.1016/j.biortech.2017.05.135
Jorge JMP, Pérez-García F, Wendisch VF (2017) A new metabolic route for the fermentative production of 5-aminovalerate from glucose and alternative carbon sources. Bioresour Technol 245:1701–1709. https://doi.org/10.1016/j.biortech.2017.04.108
Juhl K, Gathergood N, Jorgensen KA (2001) Catalytic asymmetric direct mannich reactions of carbonyl compounds with alpha-imino esters. Angew Chem Int Ed 40:2995–2997. doi: 10.1002/1521-3773(20010817)40:16<2995:AID-ANIE2995>3.0.CO;2-M
Kcomber I (2016) CCM-2016 China’s lysine market: continuation of market downturn in 2015. Kcomber Inc, Guangzhou
Kelle R, Hermann T, Bathe B (2005) l-lysine production. In: Eggeling L, Bott M (eds) Handbook of Corynebacterium glutamicum. CRC Press, Boca Raton, pp 465–488
Khaw LE, Bohm GA, Metcalfe S, Staunton J, Leadlay PF (1998) Mutational biosynthesis of novel rapamycins by a strain of Streptomyces hygroscopicus NRRL 5491 disrupted in rapL, encoding a putative lysine cyclodeaminase. J Bacteriol 180:809–814
Kim JH, Seo HM, Sathiyanarayanan G, Bhatia SK, Song HS, Kim JY, Jeon JM, Yoon JJ, Kim YG, Park K, Yang YH (2017) Development of a continuous l-lysine bioconversion system for cadaverine production. J Ind Eng Chem 46:44–48. https://doi.org/10.1016/j.jiec.2016.09.038
Kind S, Jeong WK, Schroder H, Wittmann C (2010) Systems-wide metabolic pathway engineering in Corynebacterium glutamicum for bio-based production of diaminopentane. Metab Eng 12:341–351. https://doi.org/10.1016/j.ymben.2010.03.005
Kind S, Jeong WK, Schrӧder H, Zelder O, Wittmann C (2010) Identification and elimination of the competing N-acetyldiamiopentane pathway for improved production of diaminopentane by Corynebacterium glutamicum. Appl Environ Microbiol 76:5175–5180. https://doi.org/10.1128/AEM.00834-10
Kind S, Kreye S, Wittmann C (2011) Metabolic engineering of cellular transport for overproduction of the platform chemical 1,5-diaminopentane in Corynebacterium glutamicum. Metab Eng 13:617–627. https://doi.org/10.1016/j.ymben.2011.07.006
Kind S, Becker J, Wittmann C (2013) Increased lysine production by flux coupling of the tricarboxylic acid cycle and the lysine biosynthetic pathway—metabolic engineering of the availability of succinyl-CoA in Corynebacterium glutamicum. Metab Eng 15:184–195. https://doi.org/10.1016/j.ymben.2012.07.005
Kind S, Neubauer S, Becker J, Yamamoto M, Völkert M, Abendroth GV, Zelder O, Wittmann C (2014) From zero to hero-production of bio-based nylon from renewable resources using engineered Corynebacterium glutamicum. Metab Eng 25:113–123. https://doi.org/10.1016/j.ymben.2014.05.007
Kobayashi M, Itoyama T, Mitani Y, Usni N (2011) Method for producing basic amino acid. EP1182261 B1
Krings E, Krumbach K, Bathe B, Kelle R, Wendisch VF, Sahm H, Eggeling L (2006) Characterization of myo-inositol utilization by Corynebacterium glutamicum: the stimulon, identification of transporters, and influence on l-lysine formation. J Bacteriol 188:8054–8061. https://doi.org/10.1128/JB.00935-06
Kusakabe H, Kodama K, Kuninaka A, Yoshino H, Misono H, Soda K (1980) A new antitumor enzyme, l-lysine alpha-oxidase from Trichoderma viride. Purification and enzymological properties. J Biol Chem 255:976–981
Lee GH, Hur W, Bremmon, CE, Flickinger MC (1996) Lysine production from methanol at 50 degree C using Bacillus methanolicus: modeling volume control, lysine concentration, and productivity using a three-phase continuous simulation. Biotechnol Bioeng 49: 639–653. https://doi.org/10.1002/(sici)1097-0290(19960320)49:6<639::aid-bit5>3.0.co;2-p
Liu P, Zhang H, Lv M, Hu MD, Li Z, Gao C, Xu P, Ma CQ (2014) Enzymatic production of 5-aminovalerate from l-lysine using l-lysine monooxygenase and 5-aminovaleramide amidohydrolase. Sci Rep 4:1–5. https://doi.org/10.1038/srep05657
Lu J, Meng HY, Meng ZY, Sun Y, Pribis JP, Zhu C, Li Q (2015) Epsilon aminocaproic acid reduces blood transfusion and improves the coaǵulation test after pediatric open-heart surgery: a meta-analysis of 5 clinical trials. Int J Exp Pathol 8:7978–7987. https://doi.org/10.1002/cncr.21958
Ma WC, Cao WJ, Zhang H, Chen KQ, Li Y, Ouyang P (2015) Enhanced cadaverine production from l-lysine using recombinant Escherichia coli co-overexpressing CadA and CadB. Biotechnol Lett 37:799–806. https://doi.org/10.1007/s10529-014-1753-5
Ma WC, Cao WJ, Zhang BW, Chen KQ, Liu Q, Li Y, Ouyang P (2015) Engineering a pyridoxal 5′-phosphate supply for cadaverine production by using Escherichia coli whole-cell biocatalysis. Sci Rep 5:15630. https://doi.org/10.1038/srep15630
Mahdavi HR, Arzani M, Peydayesh M, Mohammadi T (2016) Pertraction of l-lysine by supported liquid membrane using D2EHPA/M2EHPA. Chem Eng Process 106:50–58. https://doi.org/10.1016/j.cep.2016.05.004
Marcheschi RJ, Li H, Zhang KC, Noey EL, Kim S, Chaubey A, Houk KN, Liao JC (2012) A synthetic recursive “+1” pathway for carbon chain elongation. ACS Chem Biol 7:689–697. https://doi.org/10.1021/cb200313e
Matsushima Y, Hirasawa T, Shimizu H (2016) Enhancement of 1,5-diaminopentane production in a recombinant strain of Corynebacterium glutamicum by Tween 40 addition. J Gen Appl Microbiol 62:42–45. https://doi.org/10.2323/jgam.62.42
Miller DL, Rodwell VW (1971) Metabolism of basic amino acids in Pseudomonas putida. Catabolism of lysine by cyclic and acyclic intermediates. J Biol Chem 246:2758–2764
Minitsuka T, Sawai H, Hatsu M, Yamada K (2007) Metabolic engineering of Corynebacterium glutamicum for cadaverine fermentation. Biosci Biotechnol Biochem 71:2130–2135. https://doi.org/10.1271/bbb.60699
Muramatsu H, Mihara H, Goto M, Miyahara I, Hirotsu K, Kurihara T, Esaki N (2005) A new family of NAD(P)H-dependent oxidoreductases distinct from conventional Rossmann-fold proteins. J Biosci Bioeng 99:541–547. https://doi.org/10.1263/jbb.99.541
Muramatsu H, Mihara H, Yasuda M, Ueda M, Kurihara T, Esaki N (2006) Enzymatic synthesis of l-Pipecolic acid by △1-piperideine-2-carboxylate reductase from Pseudomonas putida. Biosci Biotechnol Biochem 70:2296–2298. https://doi.org/10.1271/bbb.60125
Na D, Yoo SM, Chung H, Park H, Park JH, Lee SY (2013) Metabolic engineering of Escherichia coli using synthetic small regulatory RNAs. Nat Biotechnol 31:171–174. https://doi.org/10.1002/biot.201300263
Nagai Y, Ito H, Yasueda H (2010) Amino acid production: l-lysine. In: Flickinger MC (ed) Encyclopedia of industrial biotechnology: bioprocess, bioseparation, and cell technology. Wiley, Hoboken
Nishi K, Endo S, Mori Y, Totsuka K, Hirao Y (2006) Method for producing cadaverine dicarboxylate and its use for the production of nylon. EP1482055 B1
Nishio Y, Nakamura Y, Kawarabayasi Y, Usuda Y, Kimura E, Sugimoto S, Matsui K, Yamagishi A, Kikuchi H, Ikeo K, Gojobori T (2015) Comparative complete genome sequence analysis of the amino acid replacements responsible for the thermostability of Corynebacterium efficiens. Genome Res 13:1572–1579. https://doi.org/10.1101/gr.1285603
Oh YH, Kang KH, Kwon MJ, Choi JW, Joo JC, Lee SH, Yang YH, Song BK, Kim IK, Yoon KH, Park K, Park SJ (2015) Development of engineered Escherichia coli whole-cell biocatalysts for high-level conversion of l-lysine into cadaverine. J Ind Microbiol Biotechnol 45:1481–1491. https://doi.org/10.1007/s10295-015-1678-6
Pacella E, Collini S, Pacella F, Piraino DC, Santamaria V, De Blasi RA (2010) Levobupivacaine vs. racemic bupivacaine in peribulbar anaesthesia: a randomized double blind study in ophthalmic surgery. Eur Rew Med Pharmacol Sci 14:539–544
Park SJ, Kim EY, Noh W, Park HM, Oh YH, Lee SH, Song BK, Jegal J, Lee SY (2013) Metabolic engineering of Escherichia coli for the production of 5-aminovalerate and glutarate as C5 platform chemicals. Metab Eng 16:42–47. https://doi.org/10.1016/j.ymben.2012.11.011
Park SJ, Oh YH, Noh W, Kim HY, Shin JH, Lee EG, Lee S, David Y, Baylon MG, Song BK, Jegal J, Lee SY, Lee SH (2014) High-level conversion of l-lysine into 5-aminovalerate that can be used for nylon 6,5 synthesis. Biotechnol J 9:1322–1328. https://doi.org/10.1002/biot.201400156
Pathania A, Sardesai AA (2015) Distinct paths for basic amino acid export in Escherichia coli: YbjE (LysO) mediates export of l-lysine. J Biotechnol 197:2036–2047. https://doi.org/10.1128/JB.02505-14
Pérez-García F, Peters-Wendisch P, Wendisch VF (2016) Engineering Corynebacterium glutamicum for fast production of l-lysine and l-pipecolic acid. Appl Microbiol Biotechnol 100:8075–8090. https://doi.org/10.1007/s00253-016-7682-6
Pérez-García F, Risse JM, Friehs K, Wendisch VF (2017) Fermentative production of l-pipecolic acid from glucose and alternative carbon sources. Biotechnol J 12:646–657. https://doi.org/10.1002/biot.201600646
Pukin AV, Boeriu CG, Scott EL, Sanders JPM, Franssen MCR (2010) An efficient enzymatic synthesis of 5-aminovaleric acid. J Mol Catal B Enzym 65:58–62. https://doi.org/10.1016/j.molcatb.2009.12.006
Qian ZG, Xia XX, Lee SY (2011) Metabolic engineering of Escherichia coli for the production of cadaverine: a five carbon diamine. Biotechnol Bioeng 108:93–103. https://doi.org/10.1002/cctc.201700516
Raemakers-Franken PC, Nossin PMM, Brandts PM, Wubbolts MG, Peeters WPH, Ernste S, Stefaan MA, De W, Schuermann M (2009) Biochemical synthesis of 6-aminocaproic acid. US7491520 B2
Raemakers-Franken PC, Schurmann M, Trefzer AC, Wildeman SMAD (2014) Preparation of 6-aminoc aproic acid from 5-formyl valeric acid. US0134681 A1
Revelles O, Espinosa-Urgel M, Molin S, Ramos JL (2004) The davDT operon of pseudomonas putida, involved in lysine catabolism, is induced in response to the pathway intermediate δ-aminovaleric acid. J Bacteriol 186:3439–3446. https://doi.org/10.1128/JB.186.11.3439-3446.2004
Revelles O, Espinosa-Urgel M, Fuhrer T, Sauer U, Ramos JL (2005) Multiple and interconnected pathways for l-lysine catabolism in pseudomonas putida KT2440. J Bacteriol 187:7500–7510. https://doi.org/10.1128/JB.187.21.7500-7510.2005
Revelles O, Wittich RM, Ramos JL (2007) Identification of the initial steps in d-lysine catabolism in pseudomonas putida. J Bacteriol 189:2787–2792. https://doi.org/10.1128/JB.01538-06
Rogers LMA, Rouden J, Lecomte L, Lasne MC (2003) Enantioselective decarboxylation–reprotonation of an α-amino malonate derivative as a route to optically enriched cyclic α-amino acid. Tetrahedron Lett 44:3047–3050. https://doi.org/10.1016/S0040-4039(03)00557-4
Rohles CM, Gieβelmann G, Kohlstedt M, Wittmann C, Becker J (2016) Systems metabolic engineering of Corynebacterium glutamicum for the production of the carbon-5 platform chemicals 5-aminovalerate and glutarate. Microb Cell Fact 15:154–166. https://doi.org/10.1186/s12934-016-0553-0
Schneider J, Niermann K, Wendish VF (2011) Production of the amino acids l-glutamate, l-lysine and l-arginine from arabinose by recombinant Corynebacterium glutamicum. J Biotechnol 154:191–198. https://doi.org/10.1016/j.jbiotec.2010.07.009
Shin JH, Park SH, Oh YH, Choi JW, Lee MH, Cho JS, Jeong KJ, Joo JC, Yu J, Park SJ, Lee SY (2016) Metabolic engineering of corynebacterium glutamicum for enhanced production of 5-aminovaleric acid. Microb Cell Fact 13:1–13. https://doi.org/10.1186/s12934-016-0566-8
Sigma MS, Vachal P, Jacobsen EN, (2000) A general catalyst for the asymmetric Strecker reaction. Angew Chem Int Ed 39: 1279–1281. https://doi.org/10.1002/(sici)1521-3773(20000403)39:7<1279::aid-anie1279>3.0.co;2-u
Singh SB, Zink DL, Liesch JM, Mosley RT, Dombrowski AW, Bills GF, Darkin-Rattray SJ, Schmatz DM, Goetz MA (2002) Structure and chemistry of apicidins, a class of novel cyclic tetrapeptides without a terminal α-keto epoxide as inhibitors of histone deacetylase with potent antiprotozoal activities. J Org Chem 67:815–825. https://doi.org/10.1021/jo016088w
Soksawatmaekhin W, Kuraishi A, Sakata K, Kashiwagi K, Igarashi K (2004) Excretion and uptake of cadaverine by CadB and its physiological functions in Escherichia coli. Mol Microbiol 51:1401–1412. https://doi.org/10.1046/j.1365-2958.2003.03913.x
Steen EJ, Kang YS, Bokinsky G, Hu ZH, Schirmer A, McClure A, del Cardayre SB, Keasling JD (2010) Microbial production of fatty-acid-derived fuels and chemicals from plant biomass. Nature 463:559–562. https://doi.org/10.1016/j.copbio.2013.02.028
Stefanini M, Dameshek W (1962) The hemorrhagic disorders; a clinical and therapeutic approach, 2nd edn. Grune & Stratton Inc, New York, pp 510–514
Tabor CW, Tabor H (1985) Polyamines in microorganisms. Microbiol Rev 49:81–99
Takeno S, Hori K, Ohtani S, Mimura A, Mitsuhashi S, Ikeda M (2016) l-Lysine production independent of the oxidative pentose phosphate pathway by Corynebacterium glutamicum with the Streptococcus mutans gapN gene. Metab Eng 37:1–10. https://doi.org/10.1016/j.ymben.2016.03.007
Tani Y, Miyake R, Yukami R, Dekishima Y, China H, Saito S, Kawabata H, Mihara H (2015) Functional expression of l-lysine α-oxidase from Scomber japonicus in Escherichia coli for one-pot synthesis of l-pipecolic acid from dl-lysine. Appl Microbiol Biotechnol 99:5045–5054. https://doi.org/10.1007/s00253-014-6308-0
Tateno T, Okada Y, Tsuchidate T, Tanaka T, Fukuda H, Kondo A (2009) Direct production of cadaverine from soluble starch using Corynebacterium glutamicum coexpressing α-amylase and lysine decarboxylase. Appl Microbiol Biot 82:115–121. https://doi.org/10.1007/s00253-008-1751-4
Tsotsou GE, Barbirato F (2007) Biochemical characterization of recombinant Streptomyces pristinaespiralis l-lysine cyclodeaminase. Biochimie 89:591–604. https://doi.org/10.1016/j.biochi.2006.12.008
Turk SCHJ, Kloosterman WP, Ninaber DK, Karin KPAM, Knutova J, Suir E, Schürmann M, Raemakers-Franken PC, Müller M, de Wildeman SMA, Raamsdonk LM, van der Pol R, Wu L, Temudo MF, van der Hoeven RAM, Akeroyd M, van der Stoel RE, Noorman HJ, Bovenberg RAL, Trefzer AC (2015) Metabolic engineering towards sustainable production of nylon-6. ACS synth Biol 5:65–73. https://doi.org/10.1021/acssynbio.5b00129
Unthan S, Baumgart M, Radek A, Herbst M, Siebert D, Brühl N, Bartsch A, Bott M, Wiechert W, Marin K, Hans S, Krӓmer R, Seibold G, Frunzke J, Kalinowski J, Rückert C, Wendisch VF, Noack S (2015) Chassis organism from Corynebacterium glutamicum—a top-down approach to identify and delete irrelevant gene clusters. Biotechnol J 10:290–301. https://doi.org/10.1002/biot.201400041
Vassilev I, Gießelmann G, Schwechheimer SK, Wittmann C, Virdis B, Krömer JO (2018) Anodic electro-fermentation: anaerobic production of l-lysine by recombinant Corynebacterium glutamicum. Biotechnol Bioeng. https://doi.org/10.1002/bit.26562
Wang JM, Gao DF, Yu XL, Li W, Qi QS (2015) Evolution of a chimeric aspartate kinase for l-lysine production using a synthetic RNA device. Appl Microbiol Biotechnol 99:8527–8536. https://doi.org/10.1007/s00253-015-6615-0
Weigelt S, Huber T, Hofmann F, Jost M, Ritzefeld M, Luy B, Freudenberger C, Majer Z, Vass E, Greie JC, Panella L, Kaptein B, Broxterman QB, Kessler H, Altendorf K, Hollosi M, Sewald N (2012) Synthesis and conformational analysis of efrapeptins. Chem Eur J 18:478–487. https://doi.org/10.1002/chem.201102134
Wendisch VF (2014) Microbial production of amino acids and derived chemicals: synthetic biology approaches to strain development. Curr Opin Biotechnol 30:51–58. https://doi.org/10.1016/j.copbio.2014.05.004
Wendish VF, Jorge JP, Pérez-García F, Sgobba E (2016) Updates on industrial production of amino acids using Corynebacterium glutamicum. World J Microbiol Biotechnol 32:105. https://doi.org/10.1007/s11274-016-2060-1
Xu JZ, Han M, Zhang JL, Guo YF, Zhang WG (2014) Metabolic engineering Corynebacterium glutamicum for the l-lysine production by increasing the flux into l-lysine biosynthetic pathway. Amino Acids 46:2165–2175. https://doi.org/10.1007/s00726-014-1768-1
Xu JZ, Zhang JL, Guo YF, Zai YG, Zhang WG (2013) Improvement of cell growth and l-lysine production by genetically modified Corynebacterium glutamicum during growth on molasses. J Ind Microbiol Biotechnol 40:1423–1432. https://doi.org/10.1007/s10295-013-1329-8
Ying HX, He X, Li Y, Chen KQ, Ouyang PK (2014) Optimization of culture conditions for enhanced lysine production using engineered Escherichia coli. Appl Biochem Biotechnol 172:3835–3843. https://doi.org/10.1007/s12010-014-0820-7
Ying HX, Wang J, Wang Z, Feng J, Chen KQ, Li Y, Ouyang PK (2015) Enhanced conversion of l-lysine to l-pipecolic acid using a recombinant Escherichia coli containing lysine cyclodeaminase as whole-cell biocatalys. J Mol Catal B Enzym 117:75–80. https://doi.org/10.1016/j.molcatb.2015.05.001
Zahoor A, Lindner SN, Wendisch VF (2012) Metabolic engineering of Corynebacterium glutamicum aimed at alternative carbon sources and new products. Comput Struct Biotechnol J 76:1422–1424. https://doi.org/10.5936/csbj.201210004
Zhang JW, Barajas JF, Burdu M, Ruegg TL, Dias B, Keasling JD (2016) Development of a transcription factor-based lactam biosensor. ACS Synth Biol 6:439–445. https://doi.org/10.1021/acssynbio.6b00136
Zhang JW, Barajas JF, Burdu M, Wang G, Baidoo EE, Keasling JD (2017) Application of an acyl-CoA ligase from Streptomyces aizunensis for lactam biosynthesis. ACS Synth Biol 6:884–890. https://doi.org/10.1021/acssynbio.6b00372
Zhang K, Sawaya M, Eisenberg D, Liao J (2010) Expanding metabolism for biosynthesis of nonnatural alcohols. Proc Natl Acad Sci USA 107:6234–6239. https://doi.org/10.1073/pnas.0807157106
Zhang LY, Chang SH, Wang J (2011) Synthetic biology: from the first synthetic cell to see its current situation and future development. Chinese Sci Bull 56:229–237. https://doi.org/10.1007/s11434-010-4304-z
Zhao CH, Zhou XH, Xiao Y, Wang D, Zhou Z, Yang ZX, Jiang XQ, Wang J (2016) Mechanisms of l-lysine extraction with sec-octylphenoxy acetic acid in sulfonated kerosene. J Chem Technol Biotechnol 91:2047–2055. https://doi.org/10.1002/jctb.4799
Zhou H, Vonk B, Roubos JA, Bovenberg RAL, Voigt CA (2015) Algorithmic co-optimization of genetic constructs and growth conditions: application to 6-ACA, a potential nylon-6 precursor. Nucleic Acids Res 43:10560–10570. https://doi.org/10.1093/nar/gkv1071
Zhou LB, Zeng AP (2015) Engineering a lysine-on riboswitch for metabolic control of lysine production in Corynebacterium glutamicum. ACS Synth Biol 4:1335–1340. https://doi.org/10.1021/acssynbio.5b00075
Acknowledgements
This work was supported by the National Natural Science Foundation of China (21206175 and 315014682), the Industrial Biotechnology Program of Tianjin Municipal Science and Technology Commission (14ZCZDSY00066), the Fundamental Research Funds for the Central Universities (Project No. 106112017CDJXFLX0014), and the Henan Provincial Science and technology Open cooperation projects (162106000014). This work is also partially supported by Open Funding Project of the State Key Laboratory of Bioreactor Engineering, Shanghai, China.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Cheng, J., Chen, P., Song, A. et al. Expanding lysine industry: industrial biomanufacturing of lysine and its derivatives. J Ind Microbiol Biotechnol 45, 719–734 (2018). https://doi.org/10.1007/s10295-018-2030-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-018-2030-8