Abstract
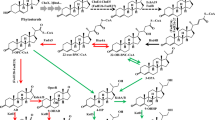
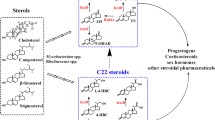
Cholesterol oxidase, steroid C27 monooxygenase and 3-ketosteroid-Δ1-dehydrogenase are key enzymes involved in microbial catabolism of sterols. Here, three isoenzymes of steroid C27 monooxygenase were firstly characterized from Mycobacterium neoaurum as the key enzyme in sterol C27-hydroxylation. Among these three isoenzymes, steroid C27 monooxygenase 2 exhibits the strongest function in sterol catabolism. To improve androst-1,4-diene-3,17-dione production, cholesterol oxidase, steroid C27 monooxygenase 2 and 3-ketosteroid-Δ1-dehydrogenase were coexpressed to strengthen the metabolic flux to androst-1,4-diene-3,17-dione, and 3-ketosteroid 9α-hydroxylase, which catalyzes the androst-1,4-diene-3,17-dione catabolism, was disrupted to block the androst-1,4-diene-3,17-dione degradation pathway in M. neoaurum JC-12. Finally, the recombinant strain JC-12S2-choM-ksdd/ΔkshA produced 20.1 g/L androst-1,4-diene-3,17-dione, which is the highest reported production with sterols as substrate. Therefore, this work is hopes to pave the way for efficient androst-1,4-diene-3,17-dione production through metabolic engineering.






Similar content being viewed by others
Abbreviations
- AD:
-
4-Androstene-3,17-dione
- ADD:
-
Androst-1,4-diene-3,17-dione
- 9α-OH-AD:
-
9α-Hydroxy-4-androstene-3,17-dione
- 9α-OH-ADD:
-
9α-Hydroxy-androst-1,4-diene-3,17-dione
- 3β-HSD:
-
3β-Hydroxysteroid dehydrogenase
- CFU:
-
Colony forming units
- ChoM:
-
Cholesterol oxidase
- SMO:
-
Steroid C27 monooxygenase
- HP-β-CD:
-
Hydroxymethyl-β-cyclodextrin
- KSDD:
-
3-Ketosteroid-Δ1-dehydrogenase
- KSH:
-
3-Ketosteroid 9α-hydroxylase
References
Bao T, Zhang X, Rao ZM, Zhao XJ, Zhang RZ, Yang TW, Xu ZH, Yang ST (2014) Efficient whole-cell biocatalyst for acetoin production with NAD+ regeneration system through homologous co-expression of 2,3-butanediol dehydrogenase and NADH oxidase in engineered Bacillus subtilis. PLoS One 9:e102951. https://doi.org/10.1371/journal.pone.0102951
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. https://doi.org/10.1016/0003-2697(76)90527-3
Brzostek A, Śliwiński T, Rumijowska-Galewicz A, Korycka-Machała M, Dziadek J (2005) Identification and targeted disruption of the gene encoding the main 3-ketosteroid dehydrogenase in Mycobacterium smegmatis. Microbiology 151:2393–2402
Capyk JK, Casabon I, Gruninger R, Strynadka NC, Eltis LD (2011) Activity of 3-ketosteroid 9α-hydroxylase (KshAB) indicates cholesterol side chain and ring degradation occur simultaneously in Mycobacterium tuberculosis. J Biol Chem 286:40717–40724
Capyk JK, Kalscheuer R, Stewart GR, Liu J, Kwon H, Zhao R, Okamoto S, Jacobs WR, Eltis LD, Mohn WW (2009) Mycobacterial cytochrome P450 125 (Cyp125) catalyzes the terminal hydroxylation of C27-steroids. J Biol Chem 284:35534–35542. https://doi.org/10.1074/jbc.M109.072132
Chaudhari P, Chaudhari B, Chincholkar S (2010) Cholesterol biotransformation to androsta-1,4-diene-3,17-dione by growing cells of Chryseobacterium gleum. Biotechnol Lett 32:695–699. https://doi.org/10.1007/s10529-010-0206-z
Chen MM, Wang FQ, Lin LC, Yao K, Wei DZ (2012) Characterization and application of fusidane antibiotic biosynthesis enzyme 3-ketosteroid-∆ 1-dehydrogenase in steroid transformation. Appl Microbiol Biotechnol 96:133–142
Chen YR, Huang HH, Cheng YF, Tang TY, Liu WH (2006) Expression of a cholesterol oxidase gene from Arthrobacter simplex in Escherichia coli and Pichia pastoris. Enzyme Microb Technol 39:854–860. https://doi.org/10.1016/j.enzmictec.2006.01.018
Chiang YR, Ismail W, Heintz D, Schaeffer C, Van Dorsselaer A, Fuchs G (2008) Study of anoxic and oxic cholesterol metabolism by Sterolibacterium denitrificans. J Bacteriol 190:905–914. https://doi.org/10.1128/Jb.01525-07
Donova MV (2007) Transformation of steroids by actinobacteria: a review. Appl Biochem Microbiol 43:1–14. https://doi.org/10.1134/s0003683807010012
Donova MV, Egorova OV (2012) Microbial steroid transformations: current state and prospects. Appl Microbiol Biotechnol 94:1423–1447. https://doi.org/10.1007/s00253-012-4078-0
Gordhan BG, Parish T (2001) Gene replacement using pretreated DNA. In: Parish T, Stoker NG (eds) Mycobacterium tuberculosis protocols, vol 54. Methods in molecular medicine. Humana Press Inc, Totowa, pp 77–92. https://doi.org/10.1385/1-59259-147-7:077
Gulla V, Banerjee T, Patil S (2010) Bioconversion of soysterols to androstenedione by Mycobacterium fortuitum subsp. fortuitum NCIM 5239, a mutant derived from total sterol degrader strain. J Chem Technol Biotechnol 85:1135–1141
Hanson JR (2005) Steroids: reactions and partial synthesis. Nat Prod Rep 22:104–110
Huang CL, Chen YR, Liu WH (2006) Production of androstenones from phytosterol by mutants of Mycobacterium sp. Enzyme Microb Technol 39:296–300
Li Y, Lu F, Sun T, Du L (2007) Expression of ksdD gene encoding 3-ketosteroid-Δ1-dehydrogenase from Arthrobacter simplex in Bacillus subtilis. Lett Appl Microbiol 44:563–568
Liu YC, Chen GY, Ge FL, Li W, Zeng L, Cao W (2011) Efficient biotransformation of cholesterol to androsta-1,4-diene-3,17-dione by a newly isolated actinomycete Gordonia neofelifaecis. World J Microbiol Biotechnol 27:759–765. https://doi.org/10.1007/s11274-010-0513-5
Mahato SB, Garai S (1997) Advances in microbial steroid biotransformation. Steroids 62:332–345
Malaviya A, Gomes J (2008) Androstenedione production by biotransformation of phytosterols. Bioresour Technol 99:6725–6737. https://doi.org/10.1016/j.biortech.2008.01.039
Molchanova MA, Andryushina VA, Savinova TS, Stytsenko TS, Rodina NV, Voishvillo NE (2007) Preparation of androsta-1,4-diene-3,17-dione from sterols using Mycobacterium neoaurum VKPM Ac-1656 strain. Russ J Bioorganic Chem 33:354–358. https://doi.org/10.1134/s1068162007030132
Ouellet H, Guan S, Johnston JB, Chow ED, Kells PM, Burlingame AL, Cox JS, Podust LM, De Montellano PRO (2010) Mycobacterium tuberculosis CYP125A1, a steroid C27 monooxygenase that detoxifies intracellularly generated cholest-4-en-3-one. Mol Microbiol 77:730–742. https://doi.org/10.1111/j.1365-2958.2010.07243.x
Ouellet H, Johnston JB, Ortiz de Montellano PR (2011) Cholesterol catabolism as a therapeutic target in Mycobacterium tuberculosis. Trends Microbiol 19:530–539. https://doi.org/10.1016/j.tim.2011.07.009
Petrusma M, Hessels G, Dijkhuizen L, van der Geize R (2011) Multiplicity of 3-ketosteroid-9α-hydroxylase enzymes in Rhodococcus rhodochrous DSM43269 for specific degradation of different classes of steroids. J Bacteriol 193:3931–3940
Rosłoniec K (2010) Steroid transformation by Rhodococcus strains and bacterial cytochrome P450 enzymes. Dissertation, University of Groningen
Shao M, Chen Y, Zhang X, Rao Z, Xu M, Yang T, Li H, Xu Z, Yang S (2017) Enhanced intracellular soluble production of 3-ketosteroid-Δ1-dehydrogenase from Mycobacterium neoaurum in Escherichia coli and its application in the androst-1,4-diene-3,17-dione production. J Chem Technol Biotechnol 92:350–357. https://doi.org/10.1002/jctb.5012
Shao ML, Rao ZM, Zhang X, Xu MJ, Yang TW, Li H, Xu ZH, Yang ST (2014) Bioconversion of cholesterol to 4-cholesten-3-one by recombinant Bacillus subtilis expressing choM gene encoding cholesterol oxidase from Mycobacterium neoaurum JC-12. J Chem Technol Biotechnol. https://doi.org/10.1002/jctb.4491
Shao ML, Rao ZM, Zhang X, Xu MJ, Yang TW, Li H, Xu ZH, Yang ST (2015) Bioconversion of cholesterol to 4-cholesten-3-one by recombinant Bacillus subtilis expressing choM gene encoding cholesterol oxidase from Mycobacterium neoaurum JC-12. J Chem Technol Biotechnol 90:1811–1820. https://doi.org/10.1002/jctb.4491
Shao ML, Zhang X, Rao ZM, Xu M, Yang T, Li H, Xu Z, Yang S (2016) A mutant form of 3-ketosteroid-Δ1-dehydrogenase gives altered androst-1,4-diene-3, 17-dione/androst-4-ene-3,17-dione molar ratios in steroid biotransformations by Mycobacterium neoaurum ST-095. J Ind Microbiol Biotechnol 43:691–701. https://doi.org/10.1007/s10295-016-1743-9
Shao ML, Zhang X, Rao ZM, Xu MJ, Yang TW, Li H, Xu ZH (2015) Enhanced production of androst-1,4-diene-3, 17-dione by Mycobacterium neoaurum JC-12 using three-stage fermentation strategy. PLoS One 10:e0137658
Shao ML, Zhang X, Rao ZM, Xu MJ, Yang TW, Li H, Xu ZH, Yang ST (2016) Efficient testosterone production by engineered Pichia pastoris co-expressing human 17β-hydroxysteroid dehydrogenase type 3 and Saccharomyces cerevisiae glucose 6-phosphate dehydrogenase with NADPH regeneration. Green Chem 18:1774–1784. https://doi.org/10.1039/c5gc02353j
Sharma P, Slathia P, Somal P, Mehta P (2012) Biotransformation of cholesterol to 1,4-androstadiene-3,17-dione (ADD) by Nocardia species. Ann Microbiol 62:1651–1659. https://doi.org/10.1007/s13213-012-0422-y
Shen YB, Wang M, Zhang LT, Ma YH, Ma B, Zheng Y, Liu H, Luo JM (2011) Effects of hydroxypropyl-β-cyclodextrin on cell growth, activity, and integrity of steroid-transforming Arthrobacter simplex and Mycobacterium sp. Appl Microbiol Biotechnol 90:1995–2003. https://doi.org/10.1007/s00253-011-3214-6
Sripalakit P, Wichai U, Saraphanchotiwitthaya A (2006) Biotransformation of various natural sterols to androstenones by Mycobacterium sp. and some steroid-converting microbial strains. J Mol Catal B Enzym 41:49–54. https://doi.org/10.1016/j.molcatb.2006.04.007
Szentirmai A (1990) Microbial physiology of sidechain degradation of sterols. J Ind Microbiol 6:101–115
Tong WY, Dong X (2009) Microbial biotransformation: recent developments on steroid drugs. Recent Pat Biotechnol 3:141–153
Van der Geize R, Yam K, Heuser T, Wilbrink MH, Hara H, Anderton MC, Sim E, Dijkhuizen L, Davies JE, Mohn WW, Eltis LD (2007) A gene cluster encoding cholesterol catabolism in a soil actinomycete provides insight into Mycobacterium tuberculosis survival in macrophages. Proc Natl Acad Sci USA 104:1947–1952. https://doi.org/10.1073/pnas.0605728104
Wang ZL, Zhao FS, Chen DJ, Li D (2006) Biotransformation of phytosterol to produce androsta-diene-dione by resting cells of Mycobacterium in cloud point system. Process Biochem 41:557–561. https://doi.org/10.1016/j.procbio.2005.09.014
Wei W, Fan SY, Wang FQ, Wei D (2010) A new steroid-transforming strain of Mycobacterium neoaurum and cloning of 3-ketosteroid 9α-hydroxylase in NwIB-01. Appl Biochem Biotechnol 162:1446–1456. https://doi.org/10.1007/s12010-010-8919-y
Wei W, Wang FQ, Fan SY, Wei DZ (2010) Inactivation and augmentation of the primary 3-ketosteroid-Δ1-dehydrogenase in Mycobacterium neoaurum NwIB-01: biotransformation of soybean phytosterols to 4-androstene-3,17-dione or 1,4-androstadiene-3,17-dione. Appl Environ Microbiol 76:4578–4582. https://doi.org/10.1128/aem.00448-10
Wilbrink MH (2011) Microbial sterol side chain degradation in Actinobacteria. University of Groningen
Wovcha MG, Antosz FJ, Knight JC, Kominek LA, Pyke TR (1978) Bioconversion of sitosterol to useful steroidal intermediates by mutants of Mycobacterium fortuitum. Biochim Biophys Acta 531:308–321. https://doi.org/10.1016/0005-2760(78)90213-8
Wovcha MG, Brooks KE, Kominek LA (1979) Evidence for two steroid 1,2-dehydrogenase activities in Mycobacterium fortuitum. Biochim Biophys Acta 574:471–479. https://doi.org/10.1016/0005-2760(79)90243-1
Xu XX, Jin FL, Yu XQ, Ji SX, Wang J, Cheng HX, Wang C, Zhang WQ (2007) Expression and purification of a recombinant antibacterial peptide, cecropin, from Escherichia coli. Protein Expr Purif 53:293–301. https://doi.org/10.1016/j.pep.2006.12.020
Yang X, Dubnau E, Smith I, Sampson NS (2007) Rv1106c from Mycobacterium tuberculosis is a 3beta-hydroxysteroid dehydrogenase. Biochemistry 46:9058–9067. https://doi.org/10.1021/bi700688x
Yao K, Wang FQ, Zhang HC, Wei DZ (2013) Identification and engineering of cholesterol oxidases involved in the initial step of sterols catabolism in Mycobacterium neoaurum. Metab Eng 15:75–87. https://doi.org/10.1016/j.ymben.2012.10.005
Yao K, Xu LQ, Wang FQ, Wei DZ (2014) Characterization and engineering of 3-ketosteroid-∆1-dehydrogenase and 3-ketosteroid-9α-hydroxylase in Mycobacterium neoaurum ATCC 25795 to produce 9α-hydroxy-4-androstene-3,17-dione through the catabolism of sterols. Metab Eng 24:181–191. https://doi.org/10.1016/j.ymben.2014.05.005
Zhang LT, Wang M, Shen YB, Ma YH, Luo JM (2009) Improvement of steroid biotransformation with hydroxypropyl-β-cyclodextrin induced complexation. Appl Biochem Biotechnol 159:642–654. https://doi.org/10.1007/s12010-008-8499-2
Zhang WQ, Shao ML, Rao ZM, Xu MJ, Zhang X, Yang TW, Li H, Xu ZH (2013) Bioconversion of 4-androstene-3,17-dione to androst-1,4-diene-3,17-dione by recombinant Bacillus subtilis expressing ksdd gene encoding 3-ketosteroid-Δ1-dehydrogenase from Mycobacterium neoaurum JC-12. J Steroid Biochem Mol Biol 135:36–42. https://doi.org/10.1016/j.jsbmb.2012.12.016
Zhang X, Zhang RZ, Bao T, Rao ZM, Yang TW, Xu MJ, Xu ZH, Li HZ, Yang ST (2014) The rebalanced pathway significantly enhances acetoin production by disruption of acetoin reductase gene and moderate-expression of a new water-forming NADH oxidase in Bacillus subtilis. Metab Eng 23:34–41. https://doi.org/10.1016/j.ymben.2014.02.002
Acknowledgements
We sincerely appreciate Professor W. R. Jacobs, Jr. (Howard Hughes Medical Institute, USA) for providing plasmids pMV261 and pMV306, and Professor T. Parish (Department of Infectious & Tropical Diseases, United Kingdom) for providing plasmids of p2NIL and pGOAL19. This work was supported by the National Natural Science Foundation of China (31700041 and 31570085), the China Post-doctoral Science Foundation Funded Project (2017M610297), the Jiangsu Province Post-doctoral Science Foundation (1701100B), The Jiangsu Province Science Fund for Distinguished Young Scholars (BK20150002), the 111 Project (111-2-06) and a Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Shao, M., Zhang, X., Rao, Z. et al. Identification of steroid C27 monooxygenase isoenzymes involved in sterol catabolism and stepwise pathway engineering of Mycobacterium neoaurum for improved androst-1,4-diene-3,17-dione production. J Ind Microbiol Biotechnol 46, 635–647 (2019). https://doi.org/10.1007/s10295-018-02135-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-018-02135-5