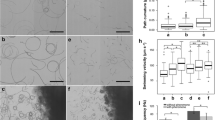
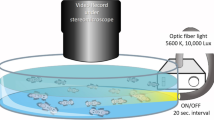
Abstract
Brown algae exhibit three patterns of sexual reproduction: isogamy, anisogamy, and oogamy. Unicellular swarmers including gametes and zoospores bear two heterogenous flagella, an anterior flagellum with mastigonemes (fine tripartite hairs) and a posterior one. In seawater, these flagellates usually receive physico-chemical signals for finding partners and good habitats. It is well known that brown algal swarmers change their swimming direction depending on blue light (phototaxis), and male gametes do so, based on the sex pheromones from female gametes (chemotaxis). In recent years, the comparative analysis of chemotaxis in isogamy, anisogamy, and oogamy has been conducted. In this paper, we focused on the phototaxis and chemotaxis of brown algal gametes comparing the current knowledge with our recent studies.






Similar content being viewed by others
References
Amsler CD, Neushul M (1989) Chemotactic effects of nutrients on spores of the kelps Macrocytis pyrifera and Pterygophora california. Mar Biol 102:557–564
Amsler CD, Neushul M (1990) Nutrient stimulation of spore settlement in the kelps Pterygophora californica and Macrocystis pyrifera. Mar Biol 107:297–304
Amsler CD, Reed DC, Neushul M (1992) The microclimate inhabited by macroalgal propagules. Br Phycol J 27:253–270
Andersen RA (2004) Biology and systematics of heterokont and haptophyte algae. Am J Bot 91:1508–1522
Baldauf SL (2003) The deep roots of eukaryotes. Science 300:1703–1706
Bouck GB (1969) Extracellular microtubules: the origin, structure, and attachment of flagellar hairs in Fucus and Ascophyllum antherozoids. J Cell Biol 40:446–460
Brokaw CJ (1979) Calcium-induced asymmetrical beating of Triton-demembranated sea urchin sperm flagella. J Cell Biol 82:401–411
Brokaw CL, Kamiya R (1987) Bending patterns of Chlamydomonas flagella: IV. Mutants with defects in inner and outer dynein arms indicate differences in dynein arm function. Cell Motil Cytoskeleton 8:68–75
Brokaw CJ, Josslin R, Bobrow L (1974) Calcium ion regulation of flagellar beat symmetry in reactivated sea urchin spermatozoa. Biochem Biophys Res Commun 58:795–800
Cardullo RA, Herrick SB, Peterson MJ, Dangott LJ (1994) Speract receptors are localized on sea urchin flagella using a fluorescent peptide analog. Dev Biol 162:600–607
Coleman AW (1988) The autofluorescent flagellum: a new phylogenetic enigma. J Phycol 24:118–120
Dring MJ (1988) Photocontrol of development in algae. Annu Rev Plant Physiol Plant Mol Biol 39:199–207
Dring MJ, Lüning K (1975) A photoperiodic response mediated by blue light in the brown alga Scytosiphon lomentaria. Planta 125:25–32
Feinleib MEH, Curry GM (1971) The relationship between stimulus intensity and oriented phototactic response (topotaxis) in Chlamydomonas. Physiol Plant 25:346–352
Fletcher RL, Callow ME (1992) The settlement, attachment and establishment of marine algal spores. Br Phycol J 27:303–329
Flores-Moya A, Posudin YI, Fernández JA, Figueroa FL, Kawai H (2002) Photomovement of the swarmers of the brown algae Scytosiphon lomentaria and Petalonia fascia: effect of photon irradiance, spectral composition and UV dose. J Photochem Photobiol B 66:134–140
Frenkel J, Vyverman W, Pohnert G (2014) Pheromone signaling during sexual reproduction in algae. Plant J 79:632–644
Fu G, Nagasato C, Ito T, Müller DG, Motomura T (2013) Ultrastructural analysis of flagellar development in plurilocular sporangia of Ectocarpus siliculosus (Phaeophyceae). Protoplasma 250:261–272
Fu G, Kinoshita N, Nagasato C, Motomura T (2014a) Fertilization of brown algae: flagellar function in phototaxis and chemotaxis. In: Sawada H, Inoue N, Iwano M (eds) Sexual reproduction in animals and plants. Springer, Tokyo, pp 359–367
Fu G, Nagasato C, Oka S, Cock JM, Motomura T (2014b) Proteomics analysis of heterogeneous flagella in brown algae (Stramenopiles). Protist 165:662–675
Fu G, Nagasato C, Yamagishi T, Kawai H, Okuda K, Takao Y, Horiguchi T, Motomura T (2016) Ubiquitous distribution of helmchrome in phototactic swarmers of the stramenopiles. Protoplasma 253:929–941
Fujita S, Iseki M, Yoshikawa S, Makino Y, Watanabe M, Motomura T, Kawai H, Murakami A (2005) Identification and characterization of a fluorescent flagellar protein from the brown alga Scytosiphon lomentaria (Scytosiphonales, Phaeophyceae): A flavoprotein homologous to Old Yellow Enzyme. Eur J Phycol 40:159–167
Geller A, Müller DG (1981) Analysis of the flagellar beat pattern of male Ectocarpus siliculosus gametes (Pheophyta) in relation to chemotactic stimulation by female cells. J Exp Biol 92:53–66
Gibbons IR (1981) Cilia and flagella of eukaryotes. J Cell Biol 91(suppl):107s–124s
Gibbons BH, Gibbons IR (1973) The effect of partial extraction of dynein arms on the movement of reactivated sea-urchin sperm. J Cell Sci 13:337–357
Glantz ST, Carpenter EJ, Melkonian M, Gardner KH, Boyden ES, Wong GK, Chow BY (2016) Functional and topological diversity of LOV domain photoreceptors. PNAS 113:E1442–E1451. doi:10.1073/pnas.1509428113
Guerrero A, Nishigaki T, Carneiro J, Yoshiro T, Wood CD, Darszon A (2010) Tuning sperm chemotaxis by calcium burst timing. Dev Biol 334:52–65
Häder D-P (1988) Ecological consequences of photomovement in microorganisms. J Photochem Photobiol B 1:385–414
Häder D-P, Colombetti G, Lenci F, Quaglia M (1981) Phototaxis in the flagellates, Euglena gracilis and Ochromonas danica. Arch Microbiol 130:78–82
Henry EC, Cole KM (1982) Ultrastructure of swarmers in the Laminariales (Phaeophyreae). I. Zoospores. J Phycol 18:550–569
Holwill MEJ, Sleigh MA (1967) Propulsion by hispid flagella. J Exp Biol 47:267–276
Inaba K (2003) Molecular architecture of the sperm flagella: molecules for motility and signaling. Zool Sci 20:1043–1056
Inaba K (2011) Sperm flagella: comparative and phylogenetic perspectives of protein components. Mol Hum Reprod 17:524–538
Jaenicke L, Starr RC (1996) The lurlenes, a new class of plastoquinone-related mating pheromones from Chlamydomonas allensworthii (Chlorophyceae). Eur J Biochem 241:581–585
Jahn TL, Landman MD, Fonseca JR (1964) The mechanism of locomotion of flagellates. II. Function of the mastigonemes of Ochromonas. J Protozool 11:291–296
Kaupp UB, Solzin J, Hildebrand E, Brown JE, Helbig A, Hagen V, Beyermann M, Pampaloni F, Weyand I (2003) The signal flow and motor response controlling chemotaxis of sea urchin sperm. Nat Cell Biol 5:109–117
Kaupp UB, Kashikar ND, Weyand I (2008) Mechanisms of sperm chemotaxis. Annu Rev Physiol 70:93–117
Kawai H (1988) A flavin-like autofluorescent substance in the posterior flagellum of golden and brown algae. J Phycol 24:114–117
Kawai H (1992a) A summary of the morphology of chloroplasts and flagellated cells in the Phaeophyceae. Korean J Phycol 7:33–43
Kawai H (1992b) Green flagellar autofluorencence in brown algal swarmers and their phototactic responses. Bot Mag Tokyo 105:171–184
Kawai H, Kreimer G (2000) Sensory mechanisms: light perception and taxis in algae. In: Leadbeater B, Green J (eds) The flagellates: unity, diversity and evolution. Taylor and Francis, London, pp 124–146
Kawai H, Müller DG, Fölster E, Häder D-P (1990) Phototactic responses in the gametes of the brown alga, Ectocarpus siliculosus. Planta 182:292–297
Kawai H, Kubota M, Kondo T, Watanabe M (1991) Action spectra for phototaxis in zoospores of the brown alga Pseudochorda gracilis. Protoplasma 161:17–22
Kinoshita N, Fu G, Ito T, Motomura T (2016a) Three-dimensional organization of flagellar basal apparatus in Ectocarpus gametes. Phycol Res 64:19–25
Kinoshita N, Nagasato C, Motomura T (2016b) Chemotactic movement in sperm of the oogamous brown alga, Saccharina japonica and Fucus distichus. Protoplasma. doi:10.1007/s00709-016-0974-y
Kinoshita N, Shiba K, Inaba K, Fu G, Nagasato C, Motomura T (2016c) Flagellar waveforms of gametes in the brown alga, Ectocarpus siliculosus. Eur J Phycol 51:139–148
Kinoshita N, Tanaka A, Nagasato C, Motomura T (2016d) Chemotaxis in the anisogamous brown alga Mutimo cylindricus. Phycologia 55:359–364
Kitayama T, Kawai H, Yoshida T (1992) Dominance of female gametophytes in field populations of Cutleria cylindrica (Cutleriales, Phaeophyceae) in the Tsugaru Strait, Japan. Phycologia 31:449–461
Kochert G (1978) Sexual pheromones in algae and fungi. Annu Rev Plant Physiol 29:461–486
Kreimer G, Kawai H, Müller DG, Melkonian M (1991) Reflective properties of the stigma in male gametes of Ectocarpus siliculosus (Phaeophyceae) studied by confocal laser scanning microscopy. J Phycol 27:268–276
Lindemann CB (2004) Testing the geometric clutch hypothesis. Biol Cell 96:681–690
Lipinska AP, D’hondt S, Damme EJV, Clerck OD (2013) Uncovering the genetic basis for early isogamete differentiation: a case study of Ectocarpus siliculosus. BMC Genom 14:909
Lipinska AP, Damme EJV, Clerck OD (2016) Molecular evolution of candidate male reproductive genes in the brown algal model Ectocarpus. BMC Evol Biol 16:5
Lüning K (1981) Egg release in gametophytes of Laminaria saccharina: induction by darkness and inhibition by blue light and UV. Br Phycol J 16:379–393
Luthringer R, Cormier A, Ahmed S, Peters AF, Cock JM, Coelho SM (2014) Sexual dimorphism in the brown algae. Perspect Phycol 1:11–25
Maier I (1993) Gamete orientation and induction of gametogenesis by pheromone in algae and plants. Plant Cell Environ 16:891–907
Maier I (1995) Brown algal pheromones. In: Round FE, Chapman DJ (eds) Progress in phycological research 11. Biopress, Bristol, pp 51–102
Maier I (1997a) The fine structure of the male gamete of Ectocarpus siliculosus (Ectocarpales, Phaeophyceae). I. General structure of the cell. Eur. J Phycol 32:241–253
Maier I (1997b) The fine structure of the male gamete of Ectocarpus siliculosus (Ectocarpales, Phaeophyceae). II. The flagellar apparatus. Eur J Phycol 32:255–266
Maier I, Calenberg M (1994) Effect of extracellular Ca2+ and Ca2+ antagonists on the movement and chemoorientation of male gametes of Ectocarpus siliculosus (Phaeophyceae). Botanica Acta 107:451–460
Maier I, Müller DG (1982) Antheridium fine structure and spermatozoid release in Laminaria digitata. Phycologia 21:1–8
Maier I, Müller DG (1986) Sexual pheromones in algae. Biol Bull 170:145–175
Maier I, Müller DG (1990) Chemotaxis in Laminaria digitata (Phaeophyceae) I. Analysis of spermatozoid movement. J Exp Bot 41:869–876
Maier I, Schmid CE (1995) An immunofluorescence study on lectin binding sites in gametes of Ectocarpus siliculosus (Ectocarpales, Phaeophyceae). Phycol Res 43: 33–42
Maier I, Wenden A, Clayton MN (1992) The movement of Hormosira banksii (Fucales, Phaeophyta) spermatozoids in response to sexual pheromone. J Exp Bot 43:1651–1657
Matsunaga S, Uchida H, Iseki M, Watanabe M, Murakami A (2010) Flagellar motions in phototactic steering in a brown algal swarmer. Photochem Photobiol B 86:374–381
Mizuno K, Shiba K, Okai M, Takahashi Y, Shitaka Y, Oiwa K, Tanokura M, Inaba K (2012) Calaxin drives sperm chemotaxis by Ca2+-mediated direct modulation of a dynein motor. Proc Natl Acad Sci USA 109:20497–20502
Morel-Laurens N (1987) Calcium control of phototactic orientation in Chlamydomorias reinhardtii: sign and strength of response. Photochem Photobiol 45:119–128
Müller DG (1977) Sexual reproduction in British Ectocarpus siliculosus (Phaeophyta). Br Phycol J 12:131–136
Müller DG (1978) Locomotive responses of male gametes to the species-specific sex attractant in Ectocarpus siliculosus (Phaeophyta). Archiv für Protistenkund 120:371–377
Müller DG (1979) Olefinic hydrocarbons in seawater: Signal molecules for sexual reproduction in brown algae. Pure Appl Chem 51:1885–1891
Müller DG, Falk H (1973) Flagellar structure of the gametes of Ectocarpus siliculosus (Phaeophyta) as revealed by negative staining. Archiv für Mikrobiol 91:313–322
Müller DG, Gassmann G, Lüning K (1979) Isolation of a spermatozoid-releasing and -attracting substance from female gametophytes of Laminaria digitata. Nature 279:430–431
Müller DG, Maier I, Müller H (1987) Flagellum autofluorescence and photoaccumulation in heterokont algae. Photochem Photobiol 46:1003–1008
Nagasato C, Motomura T, Ichimura T (1998) Selective disappearance of maternal centrioles after fertilization in the anisogamous brown alga Cutleria cylindrica (Cutleriales, Phaeophyceae): paternal inheritance of centrioles is universal in the brown algae. Phycol Res 46:191–198
Nishigaki T, Chiba K, Hoshi M (2000) A 130-kDa membrane protein of sperm flagella is the receptor for asterosaps, sperm-activating peptides of starfish Asterias amurensis. Dev Biol 219:154–162
Nozaki H, Yamada TK, Takahashi F, Matsuzaki R, Nakada T (2014) New “missing link” genus of the colonial volvocine green algae gives insights into the evolution of oogamy. BMC Evol Biol 14:37
Nultsch W, Häder D-P (1988) Photomovement in motile microorganisms. II. Photochem Photobiol 47:837–869
O’Kelly CJ (1989) The evolutionary origin of the brown algae: information from studies of motile cell structure. In: Green JC, Leadbeater BSC, Diver WL (eds) The chromophyte algae: problems and perspectives, systematics association special, 38. Clarendon Press, Oxford, pp 256–278
O’Kelly CJ, Floyd GL (1984) The absolute configuration of the flagellar apparatus in zoospores from two species of Laminariales (Phaeophyceae). Protoplasma 123:18–25
Pearson GA, Serrão EA, Dring MJ, Schmid R (2004) Blue- and green-light signals for gamete release in the brown alga, Silvetia compressa. Oecologia 138:193–201
Reed DC, Laur DR, Ebeling AW (1988) Variation in algal dispersal and recruitment: the importance of episodic events. Ecol Monogr 58:321–335
Schmid CE (1993) Cell-cell-recognition during fertilization in Ectocarpus siliculosus (Phaeophyceae). Hydrobiologia 261:437–443
Schmid CE, Schroer N, Müller DG (1994) Female gamete membrane glycoproteins potentially involved in gamete recognition in Ectocarpus siliculosus (Phaeophyceae). Plant Sci 102:61–67
Shiba K, Baba SA, Inoue T, Yoshida M (2008) Ca2+ bursts occur around a local minimal concentration of attractant and trigger sperm chemotactic response. Proc Natl Acad Sci USA 105:19312–19317
Silberfeld T, Leigh JW, Verbruggen H, Cruaud C, de Reviers B, Rousseau F (2010) A multi-locus time-calibrated phylogeny of the brown algae (Heterokonta, Ochrophyta, Phaeophyceae): Investigating the evolutionary nature of the “brown algal crown radiation”. Mol Phylogenet Evol 56:659–674
Singh S, Lowe DG, Thorpe DS, Rodriguez H, Kuang W-J, Dangott LJ, Chinkers M, Goeddel DV, Garbers DL (1988) Membrane guanylate cyclase is a cell-surface receptor with homology to protein kinases. Nature 334:708–712
Starr RC, Marner FJ, Jaenicke L (1995) Chemoattraction of male gametes by a pheromone produced by female gametes of Chlamydomonas. Proc Natl Acad Sci USA 92:641–645
Takahashi F (2016) Blue-light-regulated transcription factor, Aureochrome, in photosynthetic stramenopiles. J Plant Res 129:189–197
Takahashi T, Watanabe M (1993) Photosynthesis modulates the sign of phototaxis of wild-type Chlamydomonas reinhardtii. Effects of red background illumination and 3-(3′,4′-dichlorophenyl)-1,1-dimethylurea. FEBS Lett 336:516–520
Teves ME, Guidobaldi HA, Unates DR, Sanchez R, Miska W, Publicover SJ, Morales Garcia AA, Giojalas LC (2009) Molecular mechanism for human sperm chemotaxis mediated by progesterone. PLoS One 4:e8211
Togashi T, Bartelt JL (2011) Evolution of anisogamy and related phenomena in marine green algae. In: Togashi T, Cox PA (eds) The evolution of anisogamy: a fundamental phenomenon underlying sexual selection. Cambridge University Press, Cambridge, pp 194–242
Wakabayashi K, Misawa Y, Mochiji S, Kamiya R (2011) Reduction–oxidation poise regulates the sign of phototaxis in Chlamydomonas reinhardtii. Proc Natl Acad Sci USA 108:11280–11284
Ward GE, Brokaw CJ, Garbers DL, Vacquier VD (1985) Chemotaxis of Arbacia punctulata spermatozoa to resact, a peptide from the egg jelly layer. J Cell Biol 101:2324–2329
Wood CD, Nishigaki T, Furuta T, Baba SA, Darszon A (2005) Real-time analysis of the role of Ca2+ in flagellar movement and motility in single sea urchin sperm. J Cell Biol 169:725–731
Wynne MJ, Loiseaux S (1976) Recent advances in life history studies of the Phaeophyta. Phycologia 15:435–452
Yamagishi T, Motomura T, Nagasato C, Kato A, Kawai H (2007) A tubular mastigoneme-related protein Ocm1 isolated from the flagellum of a chromophyte alga Ochromonas danica. J Phycol 43:519–527
Yamagishi T, Motomura T, Nagasato C, Kato A, Kawai H (2009) Novel proteins comprising the stramenopile tripartite mastigoneme in Ochromonas danica (Chrysophyceae). J Phycol 45:1100–1105
Yamanouchi S (1912) The life history of Cutleria. Contr Hull Bot Lab163. Bot Gaz 54:441–502
Yoshida M, Yoshida K (2011) Sperm chemotaxis and regulation of flagellar movement by Ca2+. Mol Hum Reprod 17:457–465
Yoshida M, Inaba K, Ishida K, Morisawa M (1994) Calcium and cyclic AMP mediate sperm activation, but Ca2+ alone contributes sperm chemotaxis in ascidian, Ciona savignyi. Dev Growth Differ 36:589–595
Yoshida M, Ishikawa M, Izumi H, De Santis R, Morisawa M (2003) Store-operated calcium channel regulates the chemotactic behavior of ascidian sperm. Proc Natl Acad Sci USA 100:149–154
Acknowledgements
We sincerely thank Dr. Makoto Terauchi, Kobe University, and Dr. Tatsuya Togashi, Chiba University, for helpful discussions. We also thank Drs. Kogiku Shiba and Kazuo Inaba of the Shimoda Marine Research Center, University of Tsukuba, for their many valuable comments. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kinoshita, N., Nagasato, C. & Motomura, T. Phototaxis and chemotaxis of brown algal swarmers. J Plant Res 130, 443–453 (2017). https://doi.org/10.1007/s10265-017-0914-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10265-017-0914-8