Abstract
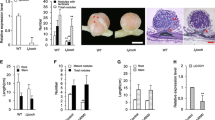
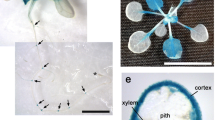
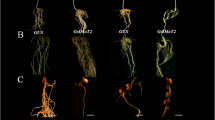
Phytohormone abscisic acid (ABA) inhibits root nodule formation of leguminous plants. LjGlu1, a β-1,3-glucanase gene of Lotus japonicus, has been identified as an ABA responsive gene. RNA interference of LjGlu1 increased nodule number. This suggests that LjGlu1 is involved in the regulation of nodule formation. Host legumes control nodule number by autoregulation of nodulation (AON), in which the presence of existing root nodules inhibits further nodulation. For further characterization of LjGlu1, we focused on the expression of LjGlu1 in relation to AON. In a split-root system, LjGlu1 expression peaked when AON was fully induced. Hairy roots transformed with LjCLE-RS1, a gene that induces AON, were generated. Expression of LjGlu1 was greater in the transgenic roots than in untransformed roots. LjGlu1 was not induced in a hypernodulating mutant inoculated with Mesorhizobium loti. These results suggest that the expression of LjGlu1 is involved in the system of AON. However, neither hypernodulation nor enlarged nodulation zone was observed on the transgenic hairy roots carrying LjGlu1-RNAi, suggesting that LjGlu1 is not a key player of AON. Recombinant LjGlu1 showed endo-β-1,3-glucanase activity. LjGlu1-mOrange fusion protein suggested that LjGlu1 associated with M. loti on the root hairs. Exogenous β-1,3-glucanase inhibited infection thread formation by both the wild type and the mutant, and nodule numbers were reduced. These results suggest that LjGlu1 is expressed in response to M. loti infection and functions outside root tissues, resulting in the inhibition of infection.








Similar content being viewed by others
References
Asamizu E, Nakamura Y, Sato S, Tabata S (2000) Generation of 7137 non-redundant expressed sequence tags from a legume, Lotus japonicus. DNA Res 7:127–130
Asamizu E, Nakamura Y, Sato S, Tabata S (2004) Characteristics of the Lotus japonicus gene repertoire deduced from large-scale expressed sequence tag (EST) analysis. Plant Mol Biol 54:405–414
Bhuvaneswari TV, Turgeon BG, Bauer WD (1980) Early events in the infection of soybean (Glycine max L. Merr.) by Rhizobium japonicum: I. Localization of infectible root cells. Plant Physiol 66:1027–1031
Boccardo G, Candresse T (2005) Complete sequence of the RNA2 of an isolate of White clover cryptic virus 1, type species of the genus Alphacryptovirus. J Arch Virol 150:403–405
Broughton WJ, Dilworth MJ (1971) Control of leghemoglobin synthesis in snake beans. Biochem J 125:1075–1080
Caetano-Anolles G, Bauer WD (1988) Feedback regulation of nodule formation in alfalfa. Planta 175:546–557
Caetano-Anolles G, Gresshoff PM (1991) Plant genetic control of nodulation. Annu Rev Microbiol 45:345–382
Cheng HP, Walker GC (1998) Succinoglycan is required for initiation and elongation of infection threads during nodulation of alfalfa by Rhizobium meliloti. J Bacteriol 180:5183–5191
Delves AC, Mathews A, Day DA, Carter AS, Carroll BJ, Gresshoff PM (1986) Regulation of the soybean-Rhizobium nodule symbiosis by shoot and root factors. Plant Physiol 82:588–590
Fåhraeus GM (1957) The infection of clover root hairs by nodule bacteria studied by a single glass slide technique. J Gen Microbiol 16:374–381
Ferguson BJ, Indrasumunar A, Hayashi S, Lin MH, Lin YH, Reid DE, Gresshoff PM (2010) Molecular analysis of legume nodule development and autoregulation. J Integr Plant Biol 52:61–76
Gerhard LM, Frederick M Jr (1999) Functions and regulation of plant β-1,3-glucanases (PR-2). In: Datta SK, Muthukrishnan S (eds) Pathogenesis-related proteins in plants, 3rd edn. Florida, Boca Raton, pp 49–76
Han SY, Kitahata N, Sekimata K, Saito T, Kobayashi M, Nakashima K, Yamaguchi-Shinozaki K, Shinozaki K, Yoshida S, Asam T (2004) A novel inhibitor of 9-cis-epoxycarotenoid dioxygenase in abscisic acid biosynthesis in higher plants. Plant Physiol 135:1574–1582
Kaneko T, Nakamura Y, Sato S, Asamizu E, Kato T, Sasamoto S, Watanabe A, Idesawa K, Ishikawa A, Kawashima K, Kimura T, Kishida Y, Kiyokawa S, Kohara M, Matsumoto M, Matsuno A, Mochizuki Y, Nakayama S, Nakazaki N, Shimpo S, Sugimoto M, Takeuchi C, Yamada M, Tabata S (2000) Complete genome structure of the nitrogen-fixing symbiotic bacterium Mesorhizobium loti. DNA Res 7:331–338
Kawaguchi M (2000) Lotus japonicus ‘Miyakojima’ MG-20: an early flowering accession suitable for indoor handing. J Plant Res 113:507–509
Kawaharada Y, Kelly S, Nielsen MW, Hjuler CT, Gysel K, Muszyński A, Carlson RW, Thygesen MB, Sandal N, Asmussen MH, Vinther M, Andersen SU, Krusell L, Thirup S, Jensen KJ, Ronson CW, Blaise M, Radutoiu S, Stougaard J (2015) Receptor-mediated exopolysaccharide perception controls bacterial infection. Nature 523:308–312
Kelly SJ, Muszyński A, Kawaharada Y, Hubber AM, Sullivan JT, Sandal N, Carlson RW, Stougaard J, Ronson CW (2013) Conditional requirement for exopolysaccharide in the Mesorhizobium-Lotus symbiosis. Mol Plant Microbe Interact 26:319–329
Kim JS, Lee J, Lee CH, Woo SY, Kang H, Seo SG, Kim SH (2015) Activation of pathogenesis-related genes by the Rhizobacterium, Bacillus sp. JS, which induces systemic resistance in tobacco plants. Plant Pathol J 31:195–201
Kosslak RM, Bohlool BB (1984) Suppression of nodule development of one side of a split-root system of soybeans caused by prior inoculation of the other side. Plant Physiol 75:125–130
Krusell L, Madsen LH, Sato S, Aubert G, Genua A, Szczyglowski K, Duc G, Kaneko T, Tabata S, de Bruijn F, Pajuelo E, Sandal N, Stougaard J (2002) Shoot control of root development and nodulation is mediated by a receptor-like kinase. Nature 420:422–426
Kumagai H, Kouchi H (2003) Gene silencing by expression of hairpin RNA in Lotus japonicus roots and root nodules. Mol Plant Microbe Interact 16:663–668
Laus MC, Logman TJ, Van Brussel AA, Carlson RW, Azadi P, Gao MY, Kijne JW (2004) Involvement of exo5 in production of surface polysaccharides in Rhizobium leguminosarum and its role in nodulation of Vicia sativa subsp. nigra. J Bacteriol 186:6617–6625
Lohar D, Stiller J, Kam J, Stacey G, Gresshoff PM (2009) Ethylene insensitivity conferred by a mutated Arabidopsis ethylene receptor gene alters nodulation in transgenic Lotus japonicus. Ann Bot 104:277–285
Maekawa T, Kusakabe M, Shimoda Y, Sato S, Tabata S, Murooka Y, Hayashi M (2008) Polyubiquitin promoter-based binary vectors for overexpression and gene silencing in Lotus japonicus. Mol Plant Microbe Interact 21:375–382
Maekawa T, Maekawa-Yoshikawa M, Takeda N, Imaizumi Anraku H, Murooka Y, Hayashi M (2009) Gibberellin controls the nodulation signaling pathway in Lotus japonicus. Plant J 58:183–194
Nakatsukasa-Akune M, Yamashita K, Shimoda Y, Uchiumi T, Abe M, Aoki T, Kamizawa A, Ayabe S, Higashi S, Suzuki A (2005) Suppression of root nodule formation by artificial expression of the TrEnodDR1 (coat protein of white clover cryptic virus 1) gene in Lotus japonicus. Mol Plant Microbe Interact 18:1069–1080
Nishimura R, Hayashi M, Wu GJ, Kouchi H, Imaizumi-Anraku H, Murakami Y, Kawasaki S, Akao S, Ohmori M, Nagasawa M, Harada K, Kawaguchi M (2002) HAR1 mediates systemic regulation of symbiotic organ development. Nature 420:426–429
Offringa IA, Melchers LS, Regensburg-Tuink AJG, Costantino P, Schilperoort RA, Hooykaas PJJ (1986) Complementation of Agrobacterium tumefaciens tumor-inducing aux mutants by genes from the TR-region of the Ri plasmid of Agrobacterium rhizogenes. Proc Natl Acad Sci USA 83:6935–6939
Oka-Kira E, Kawaguchi M (2006) Long-distance signaling to control root nodule number. Curr Opin Plant Biol 9:496–502
Okamoto S, Ohnishi E, Sato S, Takahashi H, Nakazono M, Tabata S, Kawaguchi M (2009) Nod factor/nitrate-induced CLE genes that drive HAR1-mediated systemic regulation of nodulation. Plant Cell Physiol 50:67–77
Pellock BJ, Cheng HP, Walker GC (2000) Alfalfa root nodule invasion efficiency is dependent on Sinorhizobium meliloti polysaccharides. J Bacteriol 182:4310–4318
Pierce M, Bauer WD (1983) A rapid regulatory response governing nodulation in soybean. Plant Physiol 73:286–290
Sasaki T, Suzaki T, Soyano T, Kojima M, Sakakibara H, Kawaguchi M (2014) Shoot-derived cytokinins systemically regulate root nodulation. Nat Commun 5:4983
Sato S, Kaneko T, Nakamura Y, Asamizu E, Kato T, Tabata S (2001) Structural analysis of a Lotus japonicus genome. I. Sequence features and mapping of fifty-six TAC clones which cover the 5.4 Mb regions of the genome. DNA Res 8:311–318
Shimoda Y, Shimoda-Sasakura F, Kucho K, Kanamori N, Nagata M, Suzuki A, Abe M, Higashi S, Uchiumi T (2009) Overexpression of class 1 plant hemoglobin genes enhances symbiotic nitrogen fixation activity between Mesorhizobium loti and Lotus japonicus. Plant J 57:254–263
Song Y, Chen D, Lu K, Sun Z, Zeng R (2015) Enhanced tomato disease resistance primed by arbuscular mycorrhizal fungus. Front Plant Sci 6:786
Stuurman N, Pacios Bras C, Schlaman HR, Wijfjes AH, Bloemberg G, Spaink HP (2000) Use of green fluorescent protein color variants expressed on stable broad-host-range vectors to visualize rhizobia interacting with plants. Mol Plant Microbe Interact 13:1163–1169
Suzuki A, Akune M, Kogiso M, Imagama Y, Osuki K, Uchiumi T, Higashi S, Han SY, Yoshida S, Asami T, Abe M (2004) Control of nodule number by the phytohormone abscisic acid in the roots of two leguminous species. Plant Cell Physiol 45:914–922
Suzuki A, Hara H, Kinoue T, Mikiko A, Uchiumi T, Kucho K, Higashi S, Hirsch AM, Arima S (2008a) Split-root study of autoregulation of nodulation in the model legume Lotus japonicus. J Plant Res 121:245–249
Suzuki A, Yamashita K, Ishihara M, Nakahara K, Abe M, Kucho K, Uchiumi T, Higashi S, Arima S (2008b) Enhanced symbiotic nitrogen fixation by Lotus japonicus containing an antisense β-1,3-glucanase gene. Plant Biotechnol 25:357–360
Szczyglowski K, Shaw RS, Wopereis J, Copeland S, Hamburger D, Kasiborskiet B, Dazzo FB, de Bruijn FJ (1998) Nodule organogenesis and symbiotic mutants of the model legume Lotus japonicus. Mol Plant-Microbe Interact 11:684–697
Takahara M, Magori S, Soyano T, Okamoto S, Yoshida C, Yano K, Sato S, Tabata S, Yamaguchi K, Shigenobu S, Takeda N, Suzaki T, Kawaguchi M (2013) TOO MUCH LOVE, a novel kelch repeat-containing F-box protein, functions in the long-distance regulation of the legume–Rhizobium symbiosis. Plant Cell Physiol 54:433–447
Acknowledgments
We thank Prof. Kawaguchi (National Institute for Basic Biology) for providing the seeds of har1-7 and the overexpress construct of LjCLE-RS1. We also thank the National BioResource Project Lotus/Glycine for providing the seeds of L. japonicus ‘Miyakojima’ MG-20.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Osuki, Ki., Hashimoto, S., Suzuki, A. et al. Gene expression and localization of a β-1,3-glucanase of Lotus japonicus . J Plant Res 129, 749–758 (2016). https://doi.org/10.1007/s10265-016-0811-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10265-016-0811-6