Abtract
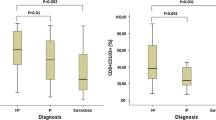
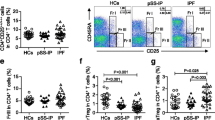
Background The pathogenetic and regulatory roles of natural killer (NK) and natural killer T-like cells in interstitial lung diseases (ILDs), fibrotic and granulomatous of unknown etiology are unclear. Objectives Here we investigated NK and NKT-like cells in peripheral blood (PB) and Bronchoalveolar lavage (BAL) from patients with ILDs. Method 190 patients (94 male mean age 61 ± 14.3 years) and 8 controls undergoing bronchoscopy for ILD diagnostic work-up were enrolled consecutively; 115 patients sarcoidosis, 24 chronic fibrotic hypersensitivity pneumonitis and 43 patients other ILDs [32 idiopathic pulmonary fibrosis (IPF) and 11 non-specific interstitial pneumonia (NSIP)]. PB and BAL were processed by flow cytometry using monoclonal antibodies to differentiate NK and NKT-like cells. Results NK% in BAL was significantly different among ILDs (p = 0.02). Lower NK% was observed in BAL from sarcoidosis than other ILDs (p < 0.05). Similar findings were observed for NKT-like, whereas no differences were found for PB NK%. Difference of NK% was observed between BAL and PB in all groups (p < 0.001). Sarcoidosis patients reported the best area under the curve for NKT-like (AUC = 0.678, p = 0.0015) and NK cells (AUC = 0.61, p = 0.001). In the IPF-NSIP subgroup, NK% cell was inversely correlated with FVC% (r = − 0.34, p = 0.03) and DLCO% (r = − 0.47, p = 0.0044). Conclusions NK and NKT-like were expressed differently in BAL from patients with different ILD and were significantly depleted in sarcoidosis respect to other ILDs. This suggests that these cells may play a protective role in the pathogenesis of sarcoidosis.




Similar content being viewed by others
Abbreviations
- LFT:
-
Lung function tests
- BAL:
-
Bronchoalveolar lavage
- NK:
-
Natural killer cells
- NKT-like:
-
Natural killer T-like cells
- IPF:
-
Idiopathic pulmonary fibrosis
- NSIP:
-
Non-specific interstitial pneumonia
- cHP:
-
Chronic fibrotic hypersensitivity pneumonitis
- ILD:
-
Interstitial lung diseases
- FVC:
-
Forced vital capacity
- FEV1:
-
Forced expiratory volume in the first second
- DLco:
-
Diffuse lung carbon monoxide
References
O’Brien KL, Finlay DK. Immunometabolism and natural killer cell responses. Nat Rev Immunol. 2019;19(5):282–90. https://doi.org/10.1038/s41577-019-0139-2.
Wang X, Peng H, Tian Z. Innate lymphoid cell memory. Cell Mol Immunol. 2019;16(5):423–9. https://doi.org/10.1038/s41423-019-0212-6.
Sivori S, Vacca P, Del Zotto G, Munari E, Mingari MC, Moretta L, Human NK. Cells: surface receptors, inhibitory checkpoints, and translational applications. Cell Mol Immunol. 2019;16(5):430–41. https://doi.org/10.1038/s41423-019-0206-4.
Jiang H-J, Wang X-X, Luo B-F, et al. Direct antiviral agents upregulate natural killer cell potential activity in chronic hepatitis C patients. Clin Exp Med. 2019;19(3):299–308. https://doi.org/10.1007/s10238-019-00564-9.
Parasa VR, Selvaraj A, Sikhamani R, Raja A. Interleukins 15 and 12 in combination expand the selective loss of natural killer T cells in HIV infection in vitro. Clin Exp Med. 2015;15(2):205–13. https://doi.org/10.1007/s10238-014-0278-5.
Shi TD, Zhang JM, Wang XF, et al. Effects of antiviral therapy with Telbivudine on peripheral iNKT cells in HBeAg(+) chronic hepatitis B patients. Clin Exp Med. 2012;12(2):105–13. https://doi.org/10.1007/s10238-011-0151-8.
Yu JC, Lin G, Field JJ, Linden J. Induction of antiinflammatory purinergic signaling in activated human iNKT cells. JCI Insight. 2018. https://doi.org/10.1172/jci.insight.91954.
Stankovic B, Bjørhovde HAK, Skarshaug R, et al. Immune cell composition in human non-small cell lung cancer. Front Immunol. 2018;9:3101. https://doi.org/10.3389/fimmu.2018.03101.
Eriksson Ström J, Pourazar J, Linder R, et al. Cytotoxic lymphocytes in COPD airways: increased NK cells associated with disease, iNKT and NKT-like cells with current smoking. Respir Res. 2018;19(1):244. https://doi.org/10.1186/s12931-018-0940-7.
Novak J, Novakova L. Prevention and treatment of type 1 diabetes mellitus by the manipulation of invariant natural killer T cells. Clin Exp Med. 2013;13(4):229–37. https://doi.org/10.1007/s10238-012-0199-0.
Lin Y-L, Lin S-C. Analysis of the CD161-expressing cell quantities and CD161 expression levels in peripheral blood natural killer and T cells of systemic lupus erythematosus patients. Clin Exp Med. 2017;17(1):101–9. https://doi.org/10.1007/s10238-015-0402-1.
Grégoire C, Chasson L, Luci C, et al. The trafficking of natural killer cells. Immunol Rev. 2007;220:169–82. https://doi.org/10.1111/j.1600-065X.2007.00563.x.
Wang J, Li F, Zheng M, Sun R, Wei H, Tian Z. Lung natural killer cells in mice: phenotype and response to respiratory infection. Immunology. 2012;137(1):37–47. https://doi.org/10.1111/j.1365-2567.2012.03607.x.
Riemann D, Cwikowski M, Turzer S, et al. Blood immune cell biomarkers in lung cancer. Clin Exp Immunol. 2019;195(2):179–89. https://doi.org/10.1111/cei.13219.
Hodge G, Hodge S. Therapeutic targeting steroid resistant pro-inflammatory NK and NKT-like cells in chronic inflammatory lung disease. Int J Mol Sci. 2019. https://doi.org/10.3390/ijms20061511.
Harpur CM, Stankovic S, Kanagarajah A, et al. Enrichment of cytomegalovirus-induced NKG2C + natural killer cells in the lung allograft. Transplantation. 2018. https://doi.org/10.1097/TP.0000000000002545.
Calabrese DR, Lanier LL, Greenland JR. Natural killer cells in lung transplantation. Thorax. 2019;74(4):397–404. https://doi.org/10.1136/thoraxjnl-2018-212345.
Souza-Fonseca-Guimaraes P, Guimaraes F, Natânia De Souza-Araujo C, et al. Natural killer cell assessment in peripheral circulation and bronchoalveolar lavage fluid of patients with severe sepsis: a case control study. Int J Mol Sci. 2017;18(3):1–2. https://doi.org/10.3390/ijms18030616.
Paget C, Trottein F. Role of type 1 natural killer T cells in pulmonary immunity. Mucosal Immunol. 2013;6(6):1054–67. https://doi.org/10.1038/mi.2013.59.
Katchar K, Söderström K, Wahlstrom J, Eklund A, Grunewald J. Characterisation of natural killer cells and CD56 + T-cells in sarcoidosis patients. Eur Respir J. 2005;26(1):77–85. https://doi.org/10.1183/09031936.05.00030805.
Papanikolaou IC, Boki KA, Giamarellos-Bourboulis EJ, et al. Innate immunity alterations in idiopathic interstitial pneumonias and rheumatoid arthritis-associated interstitial lung diseases. Immunol Lett. 2015;163(2):179–86. https://doi.org/10.1016/j.imlet.2014.12.004.
Sokhatska O, Padrão E, Sousa-Pinto B, et al. NK and NKT cells in the diagnosis of diffuse lung diseases presenting with a lymphocytic alveolitis. BMC Pulm Med. 2019;19:1–2. https://doi.org/10.1186/s12890-019-0802-1.
Travis WD, Costabel U, Hansell DM, et al. An official american thoracic society/European respiratory society statement: update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med. 2013;188(6):733–48. https://doi.org/10.1164/rccm.201308-1483ST.
Hunninghake GW, Costabel U, Ando M, et al. ATS/ERS/WASOG statement on sarcoidosis. American thoracic society/European respiratory society/World association of sarcoidosis and other granulomatous disorders. Sarcoidosis Vasc Diffuse Lung Dis Off J WASOG. 1999;16(2):149–73.
Morisset J, Johannson KA, Jones KD, et al. Identification of diagnostic criteria for chronic hypersensitivity pneumonitis: an international modified delphi survey. Am J Respir Crit Care Med. 2018;197(8):1036–44. https://doi.org/10.1164/rccm.201710-1986OC.
Culver BH, Graham BL, Coates AL, et al. Recommendations for a standardized pulmonary function report. An official American thoracic society technical statement. Am J Respir Crit Care Med. 2017;196(11):1463–72. https://doi.org/10.1164/rccm.201710-1981st.
Meyer KC, Raghu G, Baughman RP, et al. An official American thoracic society clinical practice guideline: the clinical utility of bronchoalveolar lavage cellular analysis in interstitial lung disease. Am J Respir Crit Care Med. 2012;185(9):1004–14. https://doi.org/10.1164/rccm.201202-0320ST.
Landi C, Bargagli E, Carleo A, et al. A functional proteomics approach to the comprehension of sarcoidosis. J Proteomics. 2015;128:375–87. https://doi.org/10.1016/j.jprot.2015.08.012.
Bergantini L, Bianchi F, Cameli P, et al. Prognostic biomarkers of sarcoidosis: a comparative study of serum chitotriosidase, ACE, Lysozyme, and KL-6. Dis Mark. 2019;2019:8565423. https://doi.org/10.1155/2019/8565423.
Bargagli E, Prasse A. Sarcoidosis: a review for the internist. Int Emerg Med. 2018;13(3):325–31. https://doi.org/10.1007/s11739-017-1778-6.
Lepzien R, Rankin G, Pourazar J, et al. Mapping mononuclear phagocytes in blood, lungs, and lymph nodes of sarcoidosis patients. J Leukoc Biol. 2019;105(4):797–807. https://doi.org/10.1002/JLB.5A0718-280RR.
Agostini C, Trentin L, Zambello R, et al. Phenotypical and functional analysis of natural killer cells in sarcoidosis. Clin Immunol Immunopathol. 1985;37(2):262–75.
Rijavec M, Volarevic S, Osolnik K, Kosnik M, Korosec P. Natural killer T cells in pulmonary disorders. Respir Med. 2011;105(Suppl 1):S20–5. https://doi.org/10.1016/S0954-6111(11)70006-3.
Korosec P, Rijavec M, Silar M, Kern I, Kosnik M, Osolnik K. Deficiency of pulmonary Valpha24 Vbeta11 natural killer T cells in corticosteroid-naïve sarcoidosis patients. Respir Med. 2010;104(4):571–7. https://doi.org/10.1016/j.rmed.2009.11.008.
Temprano J, Becker BA, Hutcheson PS, Knutsen AP, Dixit A, Slavin RG. Hypersensitivity pneumonitis secondary to residential exposure to Aureobasidium pullulans in 2 siblings. Ann Allergy Asthma Immunol Off Publ Am Coll Allergy Asthma Immunol. 2007;99(6):562–6. https://doi.org/10.1016/S1081-1206(10)60387-0.
Nogueira R, Melo N, e Bastos HN, et al. Hypersensitivity pneumonitis: antigen diversity and disease implications. Pulmonology. 2019;25(2):97–108. https://doi.org/10.1016/j.pulmoe.2018.07.003.
Boyton R. The role of natural killer T cells in lung inflammation. J Pathol. 2008;214(2):276–82. https://doi.org/10.1002/path.2290.
Papakosta D, Manika K, Gounari E, et al. Bronchoalveolar lavage fluid and blood natural killer and natural killer T-like cells in cryptogenic organizing pneumonia. Respirol Carlton Vic. 2014;19(5):748–54. https://doi.org/10.1111/resp.12305.
Galati D, De Martino M, Trotta A, et al. Peripheral depletion of NK cells and imbalance of the Treg/Th17 axis in idiopathic pulmonary fibrosis patients. Cytokine. 2014;66(2):119–26. https://doi.org/10.1016/j.cyto.2013.12.003.
Goh NS, Hoyles RK, Denton CP, et al. Short-term pulmonary function trends are predictive of mortality in interstitial lung disease associated with systemic sclerosis. Arthritis Rheumatol. 2017;69(8):1670–8. https://doi.org/10.1002/art.40130.
Funding
The study was conducted at Siena University without funding sponsors.
Author information
Authors and Affiliations
Contributions
LB conducted the study. LB and PC helped to define the study objectives and to coordinate the study. LB, PC and Md performed the statistical analysis and interpreted the results, CL, RMR and MP collected the data, MS and CV performed experiment, LB, EB and PS wrote the first draft of the manuscript. All authors critically revised the manuscript and approved its final version.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee (Local Ethics Committee C.E. A. V. S. E. (Code Number 180712) and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bergantini, L., Cameli, P., d’Alessandro, M. et al. NK and NKT-like cells in granulomatous and fibrotic lung diseases. Clin Exp Med 19, 487–494 (2019). https://doi.org/10.1007/s10238-019-00578-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10238-019-00578-3