Abstract
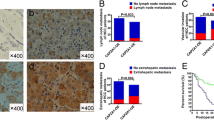
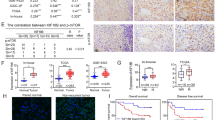
Myosin light chain kinase (MYLK) is found to catalyze the phosphorylation of myosin light chains (MLC) and regulate invasion and metastasis in some malignancies. However, there is little knowledge on the role of MYLK in hepatocellular carcinoma (HCC), and no studies have been conducted to investigate the mechanisms underlying MYLK-mediated promotion of HCC invasion and metastasis until now. In this study, we investigated the expression of MYLK in 50 pairs of human HCC and adjacent liver specimens. High MYLK expression was significantly correlated with aggressive clinicopathological features including tumor encapsulation, microvascular invasion and metastasis. In vitro assays showed that shRNA-induced MYLK knockdown significantly inhibited the wound-healing ability of HCC cells and the ability to migrate and invade through Matrigel. We next uncovered that MYLK knockdown resulted in a reduction in the number of F-actin stress fibers, disorganization of F-actin architectures and morphological alterations of HCC cells. Phosphorylated MLC, rather than total MLC, was found to be markedly reduced in response to downregulation of MYLK expression, and MYLK-regulated actin cytoskeleton through phosphorylating MLC in HCC cells. In addition, Western blotting assay revealed downregulation of the epithelial marker E-cadherin and upregulation of mesenchymal markers Vimentin, N-cadherin and Snail. Taken together, our findings indicate that MYLK promotes HCC progression by altering cytoskeleton to enhance epithelial–mesenchymal transition (EMT).





Similar content being viewed by others
Abbreviations
- MYLK:
-
Myosin light chain kinase
- HCC:
-
Hepatocellular carcinoma
- EMT:
-
Epithelial–mesenchymal transition
- MLC:
-
Myosin light chains
- IHC:
-
Immunohistochemistry
- shRNA:
-
Short hairpin RNAs
- qRT-PCR:
-
Quantitative real-time PCR
- NC:
-
Negative control
- SDS-PAGE:
-
Sodium dodecyl sulfate polyacrylamide gel electrophoresis
- IF:
-
Immunofluorescence
- SD:
-
Standard deviation
References
Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–74. https://doi.org/10.1016/j.cell.2011.02.013.
Kelley LC, Shahab S, Weed SA. Actin cytoskeletal mediators of motility and invasion amplified and overexpressed in head and neck cancer. Clin Exp Metas. 2008;25(4):289–304. https://doi.org/10.1007/s10585-008-9154-6.
Cui C, Merritt R, Fu L, Pan Z. Targeting calcium signaling in cancer therapy. Acta pharmaceutica Sinica B. 2017;7(1):3–17. https://doi.org/10.1016/j.apsb.2016.11.001.
Khapchaev AY, Shirinsky VP. Myosin Light Chain Kinase MYLK1: anatomy, Interactions, Functions, and Regulation. Biochem Biokhimiia. 2016;81(13):1676–97. https://doi.org/10.1134/S000629791613006X.
Stull JT, Tansey MG, Tang DC, Word RA, Kamm KE. Phosphorylation of myosin light chain kinase: a cellular mechanism for Ca2+ desensitization. Mol Cell Biochem. 1993;127–128:229–37.
Park J, Kim DH, Kim HN, Wang CJ, Kwak MK, Hur E, et al. Directed migration of cancer cells guided by the graded texture of the underlying matrix. Nat Mater. 2016;15(7):792–801. https://doi.org/10.1038/nmat4586.
Cui WJ, Liu Y, Zhou XL, Wang FZ, Zhang XD, Ye LH. Myosin light chain kinase is responsible for high proliferative ability of breast cancer cells via anti-apoptosis involving p38 pathway. Acta Pharmacol Sin. 2010;31(6):725–32. https://doi.org/10.1038/aps.2010.56.
Tan X, Chen M. MYLK and MYL9 expression in non-small cell lung cancer identified by bioinformatics analysis of public expression data. Tumour biology : the journal of the International Society for Oncodevelopmental Biology and Medicine. 2014;35(12):12189–200. https://doi.org/10.1007/s13277-014-2527-3.
Leveille N, Fournier A, Labrie C. Androgens down-regulate myosin light chain kinase in human prostate cancer cells. J Steroid Biochem Mol Biol. 2009;114(3–5):174–9. https://doi.org/10.1016/j.jsbmb.2009.02.002.
Chen L, Su L, Li J, Zheng Y, Yu B, Yu Y, et al. Hypermethylated FAM5C and MYLK in serum as diagnosis and pre-warning markers for gastric cancer. Dis Mark. 2012;32(3):195–202. https://doi.org/10.3233/DMA-2011-0877.
Choi C, Kwon J, Lim S, Helfman DM. Integrin beta1, myosin light chain kinase and myosin IIA are required for activation of PI3 K-AKT signaling following MEK inhibition in metastatic triple negative breast cancer. Oncotarget. 2016;7(39):63466–87. https://doi.org/10.18632/oncotarget.11525.
Sundararajan V, Gengenbacher N, Stemmler MP, Kleemann JA, Brabletz T, Brabletz S. The ZEB1/miR-200c feedback loop regulates invasion via actin interacting proteins MYLK and TKS5. Oncotarget. 2015;6(29):27083–96. https://doi.org/10.18632/oncotarget.4807.
Zuo L, Yang X, Lu M, Hu R, Zhu H, Zhang S, et al. All-Trans Retinoic Acid Inhibits Human Colorectal Cancer Cells RKO Migration via Downregulating Myosin Light Chain Kinase Expression through MAPK Signaling Pathway. Nutr Cancer. 2016;68(7):1225–33. https://doi.org/10.1080/01635581.2016.1216138.
Kim DY, Helfman DM. Loss of MLCK leads to disruption of cell-cell adhesion and invasive behavior of breast epithelial cells via increased expression of EGFR and ERK/JNK signaling. Oncogene. 2016;35(34):4495–508. https://doi.org/10.1038/onc.2015.508.
Zhang X, Yu H. Matrine inhibits diethylnitrosamine-induced HCC proliferation in rats through inducing apoptosis via p53, Bax-dependent caspase-3 activation pathway and down-regulating MLCK overexpression. Iran J Pharm Res IJPR. 2016;15(2):491–9.
Blue EK, Goeckeler ZM, Jin Y, Hou L, Dixon SA, Herring BP, et al. 220- and 130-kDa MLCKs have distinct tissue distributions and intracellular localization patterns. Am J Physiol Cell Physiol. 2002;282(3):C451–60. https://doi.org/10.1152/ajpcell.00333.2001.
Yokota J. Tumor progression and metastasis. Carcinogenesis. 2000;21(3):497–503.
Iwatsuki M, Mimori K, Yokobori T, Ishi H, Beppu T, Nakamori S, et al. Epithelial-mesenchymal transition in cancer development and its clinical significance. Cancer Sci. 2010;101(2):293–9. https://doi.org/10.1111/j.1349-7006.2009.01419.x.
Yu HJ, Serebryannyy LA, Fry M, Greene M, Chernaya O, Hu WY, et al. Tumor Stiffness Is Unrelated to Myosin Light Chain Phosphorylation in Cancer Cells. PLoS ONE. 2013;8(11):e79776. https://doi.org/10.1371/journal.pone.0079776.
Zou DB, Wei X, Hu RL, Yang XP, Zuo L, Zhang SM, et al. Melatonin inhibits the migration of colon cancer RKO cells by down-regulating myosin light chain kinase expression through cross-talk with p38 MAPK. Asian Pacific journal of cancer prevention : APJCP. 2015;16(14):5835–42.
Wang B, Yan Y, Zhou J, Zhou Q, Gui S, Wang Y, Wang Y. A novel all-trans retinoid acid derivatives inhibits the migration of breast cancer cell lines MDA-MB-231 via myosin light chain kinase involving p38-MAPK pathway. Biomed Pharmacother. 2013;67(5):357–62. https://doi.org/10.1016/j.biopha.2013.03.016.
Yilmaz M, Christofori G. Mechanisms of motility in metastasizing cells. Mol Cancer Res MCR. 2010;8(5):629–42. https://doi.org/10.1158/1541-7786.MCR-10-0139.
Olson MF, Sahai E. The actin cytoskeleton in cancer cell motility. Clin Exp Metas. 2009;26(4):273–87. https://doi.org/10.1007/s10585-008-9174-2.
Gross SR. Actin binding proteins: their ups and downs in metastatic life. Cell Adhes Migr. 2013;7(2):199–213. https://doi.org/10.4161/cam.23176.
Yilmaz M, Christofori G. EMT, the cytoskeleton, and cancer cell invasion. Cancer Metastasis Rev. 2009;28(1–2):15–33. https://doi.org/10.1007/s10555-008-9169-0.
Zeisberg M, Neilson EG. Biomarkers for epithelial-mesenchymal transitions. J Clin Investig. 2009;119(6):1429–37. https://doi.org/10.1172/JCI36183.
Acknowledgements
This study was funded by the National Natural Science Foundation of China (Grant Number: ‘81301444’), the Special Fund of Fujian Provincial Department of Finance (Grant Number: ‘2014-1262’) and the Research Talent Training Program of Fujian Provincial Health and Family Planning Commission (Grant Number: ‘2017-ZQN-14’).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Lin, J., He, Y., Chen, L. et al. MYLK promotes hepatocellular carcinoma progression through regulating cytoskeleton to enhance epithelial–mesenchymal transition. Clin Exp Med 18, 523–533 (2018). https://doi.org/10.1007/s10238-018-0509-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10238-018-0509-2