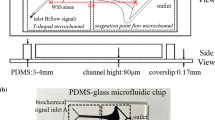
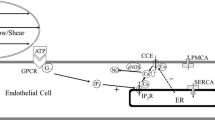
Abstract
Revealing the mechanisms underlying the intracellular calcium responses in vascular endothelial cells (VECs) induced by mechanical stimuli contributes to a better understanding for vascular diseases, including hypertension, atherosclerosis, and aneurysm. Combining with experimental measurement and Computational Fluid Dynamics simulation, we developed a mechanobiological model to investigate the intracellular [Ca2+] response in a single VEC being squeezed through narrow microfluidic channel. The time-dependent cellular surface tension dynamics was quantified throughout the squeezing process. In our model, the various Ca2+ signaling pathways activated by mechanical stimulation is fully considered. The simulation results of our model exhibited well agreement with our experimental results. By using the model, we theoretically explored the mechanism of the two-peak intracellular [Ca2+] response in single VEC being squeezed through narrow channel and made some testable predictions for guiding experiment in the future.









Similar content being viewed by others
References
Barakat AI (2001) A model for shear stress-induced deformation of a flow sensor on the surface of vascular endothelial cells. J Theor Biol 210:221–236
Barakat AI, Lieu DK, Gojova A (2006) Secrets of the code: do vascular endothelial cells use ion channels to decipher complex flow signals? Biomaterials 27:671–678
Brohawn SG, Su Z, Mackinnon R (2014) Mechanosensitivity is mediated directly by the lipid membrane in TRAAK and TREK1 K+ channels. PNAS 111:3614–3619
Brugues J, Maugis B, Casademunt J, Nassoy P, Amblard F, Sens P (2010) Dynamical organization of the cytoskeletal cortex probed by micropipette aspiration. PNAS 107:15415–15420
Chen ZZ, Gao ZM, Zeng DP, Liu B, Luan Y, Qin KR (2016) A Y-shaped microfluidic device to study the combined effect of wall shear stress and ATP signals on intracellular calcium dynamics in vascular endothelial cells. Micromachines 7:213. https://doi.org/10.3390/mi7110213
Chen ZZ, Yuan WM, Xiang C, Zeng DP, Liu B, Qin KR (2019) A microfluidic device with spatiotemporal wall shear stress and ATP signals to investigate the intracellular calcium dynamics in vascular endothelial cells. Biomech Model Mechanobiol 18:189–202. https://doi.org/10.1007/s10237-018-1076-x
Coughlin MF, Bielenberg DR, Lenormand G, Marinkovic M, Waghorne C, Zetter BR, Fredberg JJ (2013) Cytoskeletal stiffness, friction, and fluidity of cancer cell lines with different metastatic potential. Clin Exp Metas 30:237–250
Dancu MB, Tarbell JM (2006) Large negative stress phase angle (SPA) attenuates nitric oxide production in bovine aortic endothelial cells. J Biomech Eng 128:329–334
Davies PF (1995) Flow-mediated endothelial mechanotransduction. Physiol Rev 75:519–560
Derganc J, Božic B, Svetina S, Žeks B (2000) Stability analysis of micropipette aspiration of neutrophils. Biophys J 79:153–162
Drury JL, Dembo M (1999) Hydrodynamics of micropipette aspiration. Biophys J 76:110–128
Everaerts W, Nilius B, Owsianik G (2010) The vanilloid transient receptor potential channel TRPV4: from structure to disease. Prog Biophys Mol Biol 103:2–17
Fernandes J, Lorenzo IM, Andrade YN, Garciaelias A, Serra SA, Fernandezfernandez JM, Valverde MA (2008) IP3 sensitizes TRPV4 channel to the mechano- and osmotransducing messenger 5′-6′-epoxyeicosatrienoic acid. J Cell Biol 181:143–155
Fung YC, Skalak R (1981) Biomechanics: mechanical properties of living tissues. J Biomech Eng 103:231–298
Gauthier NC, Rossier O, Mathur A, Hone J, Sheetz MP (2009) Plasma membrane area increases with spread area by exocytosis of a GPI-anchored protein compartment. Mol Biol Cell 20:3261–3272
Gauthier NC, Fardin M, Rocacusachs P, Sheetz MP (2011) Temporary increase in plasma membrane tension coordinates the activation of exocytosis and contraction during cell spreading. PNAS 108:14467–14472
Gauthier NC, Masters TA, Sheetz MP (2012) Mechanical feedback between membrane tension and dynamics. Trends Cell Biol 22:527–535
Grygorczyk R, Hanrahan JW (1997) CFTR-independent ATP release from epithelial cells triggered by mechanical stimuli. Am J Physiol Cell Physiol 272:C1058–C1066
Houk AR et al (2012) Membrane tension maintains cell polarity by confining signals to the leading edge during neutrophil migration. Cell 148:175–188
Hu XQ, Xiang C, Cao LL, Xu Z, Qin KR (2008) A mathematical model for ATP-mediated calcium dynamics in vascular endothelial cells induced by fluid shear stress. Appl Math Mech 29:1291–1298
Keren K, Pincus Z, Allen GM, Barnhart EL, Marriott G, Mogilner A, Theriot JA (2008) Mechanism of shape determination in motile cells. Nature 453:475–480
Kumar S, Weaver VM (2009) Mechanics, malignancy, and metastasis: the force journey of a tumor cell. Cancer Metastasis Rev 28:113–127
Kung C (2005) A possible unifying principle for mechanosensation. Nature 436:647–654
Lee CH, Shin HJ, Cho IH, Kang Y, Kim IA, Park KD, Shin J (2005) Nanofiber alignment and direction of mechanical strain affect the ECM production of human ACL fibroblast. Biomaterials 26:1261–1270
Li LF, Xiang C, Qin KR (2015) Modeling of TRPV4-C1-mediated calcium signaling in vascular endothelial cells induced by fluid shear stress and ATP. Biomech Model Mechanobiol 14:979
Needham D, Hochmuth RM (1992) A sensitive measure of surface stress in the resting neutrophil. Biophys J 61:1664–1670
Nieponice A, Maul TM, Cumer JM, Soletti L, Vorp DA (2007) Mechanical stimulation induces morphological and phenotypic changes in bone marrow-derived progenitor cells within a three-dimensional fibrin matrix. J Biomed Mater Res Part A 81:523–530
Nilius B, Honore E (2012) Sensing pressure with ion channels. Trends Neurosci 35:477–486
Pachenari M, Seyedpour SM, Janmaleki M, Shayan SB, Taranejoo S, Hosseinkhani H (2014) Mechanical properties of cancer cytoskeleton depend on actin filaments to microtubules content: investigating different grades of colon cancer cell lines. J Biomech 47:373–379
Pedersen SF, Nilius B (2007) Transient receptor potential channels in mechanosensing and cell volume regulation. Methods Enzymol 428:183–207
Peng X, Recchia FA, Byrne BJ, Wittstein IS, Ziegelstein RC, Kass DA (2000) In vitro system to study realistic pulsatile flow and stretch signaling in cultured vascular cells. Am J Physiol Cell Physiol 279:C797–C805
Peukes J, Betz T (2014) Direct measurement of the cortical tension during the growth of membrane blebs. Biophys J 107:1810–1820
Plank MJ, Wall D, David T (2006) Atherosclerosis and calcium signalling in endothelial cells. Prog Biophys Mol Biol 91:287–313
Putney JW, Broad LM, Braun F, Lievremont J, Bird GS (2001) Mechanisms of capacitative calcium entry. J Cell Sci 114:2223–2229
Qin KR, Xiang C, Xu Z, Cao LL, Ge SS, Jiang ZL (2008) Dynamic modeling for shear stress induced ATP release from vascular endothelial cells. Biomech Model Mechanobiol 7:345–353
Qin KR, Xiang C, Cao LL (2011) Dynamic modeling for flow-activated chloride-selective membrane current in vascular endothelial cells. Biomech Model Mechanobiol 10:743–754
Sens P, Turner MS (2006) Budded membrane microdomains as tension regulators. Phys Rev E 73:031918
Sonkusare SK et al (2012) Elementary Ca2+ signals through endothelial TRPV4 channels regulate vascular function. Science 336:597–601
Sprague RS, Ellsworth ML, Stephenson AH, Kleinhenz ME, Lonigro AJ (1998) Deformation-induced ATP release from red blood cells requires CFTR activity. Am J Physiol Heart Circ Physiol 275:H1726–H1732
White JPM, Cibelli M, Urban L, Nilius B, Mcgeown JG, Nagy I (2016) TRPV4: molecular conductor of a diverse orchestra. Physiol Rev 96:911–973
Wiesner TF, Berk BC, Nerem RM (1997) A mathematical model of the cytosolic-free calcium response in endothelial cells to fluid shear stress. PNAS 94:3726–3731
Yamamoto K, Korenaga R, Kamiya A, Ando J (2000) Fluid shear stress activates Ca2+ influx into human endothelial cells via P2X4 purinoceptors. Circ Res 87:385–391
Yamamoto K, Sokabe T, Ohura N, Nakatsuka H, Kamiya A, Ando J (2003) Endogenously released ATP mediates shear stress-induced Ca2+ influx into pulmonary artery endothelial cells. Am J Physiol Heart Circ Physiol 285:H793–H803
Yamamoto K et al (2006) Impaired flow-dependent control of vascular tone and remodeling in P2X4-deficient mice. Nat Med 12:133–137
Yeung A, Evans E (1989) Cortical shell-liquid core model for passive flow of liquid-like spherical cells into micropipets. Biophys J 56:139–149
Yuan WM, Shao JY, Xue CD, Liu B, Qin KR (2019a) A high-throughput microfluidic device for probing calcium dynamics of single cells squeezing through narrow channels. J Micromech Microeng 29:115014
Yuan WM, Xiang C, Xue CD, Liu B, Qin KR (2019b) Measuring the apparent viscosities of single cells by tracking the entire deformation dynamics in microfluidic channels. Anal Methods 11:5680–5690
Zhelev DV, Hochmuth RM (1995) Mechanically stimulated cytoskeleton rearrangement and cortical contraction in human neutrophils. Biophys J 68:2004–2014
Acknowledgements
The research described in this paper was, in part, supported by the National Natural Science Foundation of China (Grant Nos. 11672065, 31371243) and the Fundamental Research Funds for the Central Universities in China (Grant No. DUT19ZD201).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yuan, WM., Xue, CD. & Qin, KR. The intracellular calcium dynamics in a single vascular endothelial cell being squeezed through a narrow microfluidic channel. Biomech Model Mechanobiol 20, 55–67 (2021). https://doi.org/10.1007/s10237-020-01368-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10237-020-01368-7