Abstract
Knowledge of the complete three-dimensional (3D) mechanical behavior of soft tissues is essential in understanding their pathophysiology and in developing novel therapies. Despite significant progress made in experimentation and modeling, a complete approach for the full characterization of soft tissue 3D behavior remains elusive. A major challenge is the complex architecture of soft tissues, such as myocardium, which endows them with strongly anisotropic and heterogeneous mechanical properties. Available experimental approaches for quantifying the 3D mechanical behavior of myocardium are limited to preselected planar biaxial and 3D cuboidal shear tests. These approaches fall short in pursuing a model-driven approach that operates over the full kinematic space. To address these limitations, we took the following approach. First, based on a kinematical analysis and using a given strain energy density function (SEDF), we obtained an optimal set of displacement paths based on the full 3D deformation gradient tensor. We then applied this optimal set to obtain novel experimental data from a 1-cm cube of post-infarcted left ventricular myocardium. Next, we developed an inverse finite element (FE) simulation of the experimental configuration embedded in a parameter optimization scheme for estimation of the SEDF parameters. Notable features of this approach include: (i) enhanced determinability and predictive capability of the estimated parameters following an optimal design of experiments, (ii) accurate simulation of the experimental setup and transmural variation of local fiber directions in the FE environment, and (iii) application of all displacement paths to a single specimen to minimize testing time so that tissue viability could be maintained. Our results indicated that, in contrast to the common approach of conducting preselected tests and choosing an SEDF a posteriori, the optimal design of experiments, integrated with a chosen SEDF and full 3D kinematics, leads to a more robust characterization of the mechanical behavior of myocardium and higher predictive capabilities of the SEDF. The methodology proposed and demonstrated herein will ultimately provide a means to reliably predict tissue-level behaviors, thus facilitating organ-level simulations for efficient diagnosis and evaluation of potential treatments. While applied to myocardium, such developments are also applicable to characterization of other types of soft tissues.













Similar content being viewed by others
Notes
The tensor B satisfies \(B_{{ijkl}} =B_{{klij}} =B_{{jikl}} =B_{{ijlk}} =B_{{jilk}} \).
References
Atwood CL (1969) Optimal and efficient designs of experiments. Ann Math Stat 40:1570–1602
Avazmohammadi R, Hill MR, Simon MA, Zhang W, Sacks MS (2016) A novel constitutive model for passive right ventricular myocardium: evidence for myofiber-collagen fiber mechanical coupling. Biomech Model Mechanobiol 16(2):561–581
Beck JV, Arnold KJ (1977) Parameter estimation in engineering and science. Wiley, Hoboken, NJ
Chabiniok R et al (2016) Multiphysics and multiscale modelling, data-model fusion and integration of organ physiology in the clinic: ventricular cardiac mechanics. Interface focus 6:20150083
Costa KD, Holmes JW, MeCulloch AD (2001) Modelling cardiac mechanical properties in three dimensions. Phil Trans R Soc Lond 359:1233–1250
Dokos S, LeGrice IJ, Smaill BH, Kar J, Young AA (2000) A triaxial-measurement shear-test device for soft biological tissues. J Biomech Eng 122:471–478
Dokos S, Smaill BH, Young AA, LeGrice IJ (2002) Shear properties of passive ventricular myocardium. Am J Physiol Heart Circ Physiol 283:H2650–2659
Freed A, Srinivasa A (2015) Logarithmic strain and its material derivative for a QR decomposition of the deformation gradient. Acta Mech 226:2645–2670
Fung YC (1993) Biomechanics: mechanical properties of living tissues, 2nd edn. Springer, New York
Göktepe S, Acharya S, Wong J, Kuhl E (2011) Computational modeling of passive myocardium. Int J Numer Methods Biomed Eng 27:1–12
Green AE, Adkins JE (1960) Large elastic deformations and non-linear continuum mechanics. Clarendon Press, Wotton-under-Edge
Guccione JM, McCulloch AD, Waldman LK (1991) Passive material properties of intact ventricular myocardium determined from a cylindrical model. J Biomech Eng 113:42–55
Gupta KB, Ratcliffe MB, Fallert MA, Edmunds L, Bogen DK (1994) Changes in passive mechanical stiffness of myocardial tissue with aneurysm formation. Circulation 89:2315–2326
Holzapfel GA, Ogden RW (2008) On planar biaxial tests for anisotropic nonlinearly elastic solids. A continuum mechanical framework. Math Mech Solids 14:474–489
Holzapfel GA, Ogden RW (2009) Constitutive modelling of passive myocardium: a structurally based framework for material characterization. Philos Trans A Math Phys Eng Sci 367:3445–3475. doi:10.1098/rsta.2009.0091
Hoppin F, Lee G, Dawson S (1975) Properties of lung parenchyma in distortion. J Appl Physiol 39:742–751
Humphrey J, Yin F (1987) A new constitutive formulation for characterizing the mechanical behavior of soft tissues. Biophys J 52:563–570
Humphrey JD, Strumpf RK, Yin FC (1990a) Determination of a constitutive relation for passive myocardium: I. A new functional form. J Biomech Eng 112:333–339
Humphrey JD, Strumpf RK, Yin FC (1990b) Determination of a constitutive relation for passive myocardium: II. Parameter estimation. J Biomech Eng 112:340–346
Intrigila B, Melatti I, Tofani A, Macchiarelli G (2007) Computational models of myocardial endomysial collagen arrangement. Comput Methods Programs Biomed 86:232–244. doi:10.1016/j.cmpb.2007.03.004
Lanir Y, Lichtenstein O, Imanuel O (1996) Optimal design of biaxial tests for structural material characterization of flat tissues. J Biomech Eng 118:41–47
LeGrice IJ, Smaill B, Chai L, Edgar S, Gavin J, Hunter PJ (1995) Laminar structure of the heart: ventricular myocyte arrangement and connective tissue architecture in the dog. Am J Physiol Heart Circul Physiol 269:H571–H582
Mehrabadi MM, Cowin SC (1990) Eigentensors of linear anisotropic elastic materials. Q J Mech Appl Math 43:15–41
Morita M et al (2011) Modification of infarct material properties limits adverse ventricular remodeling. Ann Thorac Surg 92:617–624
Nathanson MH, Saidel GM (1985) Multiple-objective criteria for optimal experimental design: application to ferrokinetics. Am J Physiol Regul Integr Comp Physiol 248:R378–R386
Ogden RW (1997) Non-linear elastic deformations. Courier Corporation, North Chelmsford
Pukelsheim F (2006) Optimal design of experiments. SIAM, Philadelphia
Rivlin RS, Saunders D (1951) Large elastic deformations of isotropic materials. VII. Experiments on the deformation of rubber. Philos Trans R Soc Lond A Math Phys Eng Sci 243:251–288
Robinson TF, Cohen-Gould L, Factor SM (1983) Skeletal framework of mammalian heart muscle. Arrangement of inter-and pericellular connective tissue structures. Lab Invest 49:482–498
Robinson TF, Factor SM, Sonnenblick EH (1986) The heart as a suction pump. Sci Am 254:84–91
Sacks MS, Chuong CJ (1993) A constitutive relation for passive right-ventricular free wall myocardium. J Biomech 26:1341–1345
Sacks MS, Smith DB, Hiester ED (1997) A small angle light scattering device for planar connective tissue microstructural analysis. Ann Biomed Eng 25:678–689
Schmid H, Nash MP, Young AA, Hunter PJ (2006) Myocardial material parameter estimation—a comparative study for simple shear. J Biomech Eng 128:742–750. doi:10.1115/1.2244576
Schmid H O’Callaghan P, Nash MP, Lin W, LeGrice IJ, Smaill BH, Young AA, Hunter PJ (2008) Myocardial material parameter estimation: a non-homogeneous finite element study from simple shear tests. Biomech Model Mechanobiol 7:161–173. doi:10.1007/s10237-007-0083-0
Scollan DF, Holmes A, Winslow R, Forder J (1998) Histological validation of myocardial microstructure obtained from diffusion tensor magnetic resonance imaging. Heart Circul Physiol 275:H2308–H2318
Sommer G, Schriefl AJ, Andrä M, Sacherer M, Viertler C, Wolinski H, Holzapfel GA (2015) Biomechanical properties and microstructure of human ventricular myocardium. Acta Biomater 24:172–192
Srinivasa A (2012) On the use of the upper triangular (or QR) decomposition for developing constitutive equations for Green-elastic materials. Int J Eng Sci 60:1–12
Vossoughi J, Vaishnav RN, Patel DJ (1980) Compressibility of myocardial tissue. In: 1980 ASME advances in bioengineering, pp 45–48
Yin FC, Strumpf RK, Chew PH, Zeger SL (1987) Quantification of the mechanical properties of noncontracting canine myocardium under simultaneous biaxial loading. J Biomech 20:577–589
Young AA, Legrice IJ, Young MA, Smaill BH (1998) Extended confocal microscopy of myocardial laminae and collagen network. J Microsc 192:139–150
Acknowledgements
This work was supported in part by the US National Institutes of Health grants 1F32 HL132543 to R.A., and T32 EB007507 to D.S.L. We’d like to thank John Lesicko for helping in the development of the TRIAX device, MaiQuyen Nguyen for assistance in histological analysis of the post-infarcted myocardium specimen, and Samarth Raut for the development of the initial FE models.
Author information
Authors and Affiliations
Corresponding author
Appendices
Appendix A: Validation of the analysis pipeline
In this appendix, we provide the results for the validation of the simulation-experimental pipeline using isotropic and anisotropic synthetic specimens as described in Sect. 2.6.2.
1.1 Isotropic gel
Single tension and simple shear tests with flat plate attachment on the same specimen (see Fig. 5b) led to matched properties. The FE model (see Fig. 5c) equipped with (single-parameter) neo-Hookean material description provided reliable fit to the stress–stretch data of single tension test (see Fig. 12a) with \(r^{2}=0.99\). The estimated value of unknown shear modulus was 35.77 kPa. This value was used in FE simulations to compare against the simple shear test date, and an overall good agreement was found with \(r^{2}=0.96\) (Fig. 12b). The agreement was particularly good for smaller deformations (up to 0.04 shear).
1.2 Transversely isotropic rubber
The FE model (see Fig. 5f) equipped with transversely isotropic Fung material model (Eq. 26) was used to fit the stress–stretch data from the first set of tests comprising of two single tension tests and two simple shear tests in the transverse and longitudinal directions. The overall fit was very good (Fig. 13a, b) with \(r^{2}=0.97\). The FE model with the estimated values (Table 5) were used to compare against the data from the second set of tests comprising of equibiaxial test in longitudinal and transverse directions, the perpendicular simple shear, and the single compression in the transverse direction (Fig. 13c, d, e). The overall fit was satisfactory to validate the robustness of the device for mechanical testing of anisotropic materials. This result also underscores how optimal design of experiments can be used to derive model parameters with good predictive capabilities.
Appendix B
In this appendix, we discuss a simple example of optimal design of mechanical testing to characterize the mechanical behavior of a transversely isotropic solid based on a three-parameter constitutive model. Our attention is restricted to D-optimality criterion and we show how this criterion can guide the experiments to optimally estimate the material constants and avoid identifiability issues. We assume that the solid material with preferred direction along \(b_3\) is characterized by following energy function
where \(c_1 \) and \(c_3 \) characterize the in-plane and out-of-plane stiffness of the material, respectively. In a similar manner as discussed in Sect. 2.4, we use the objective function \(\varPhi \) to estimate the parameters. To be able to visualize the objective function in three-dimensional space, we assign a value to \(c_0 \) and use mechanical tests to estimate \(c_1 \) and \(c_3 \). We compare the values of the D-optimality criterion \(\varDelta _\mathrm{D} \), defined in (20), for three sets each of which is composed of two deformation protocols as follows:
-
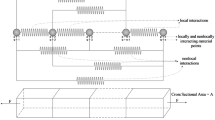
Set 1: Simple shear XY and simple shear ZX (see Fig. 2)
-
Set 2: Single tension X and simple shear ZX
-
Set 3: Single tension X and single tension Z
We calculated the objective function for numerical values \(c_0 =3 \hbox { kPa}\), \(c_1 =2\), and \(c_3 =5\) (Fig. 14). A zero value for \(\varDelta _\mathrm{D} \) indicates that \(c_1\) and \(c_3\) are not uniquely identifiable (Fig. 14a) through Set 1, and the increasing value of \(\varDelta _\mathrm{D} \) for Sets 2 and 3 reflects the modifying stability of the optimization process (Fig. 14b, c).
Rights and permissions
About this article
Cite this article
Avazmohammadi, R., Li, D.S., Leahy, T. et al. An integrated inverse model-experimental approach to determine soft tissue three-dimensional constitutive parameters: application to post-infarcted myocardium. Biomech Model Mechanobiol 17, 31–53 (2018). https://doi.org/10.1007/s10237-017-0943-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10237-017-0943-1