Abstract
The reproductive system of the red stingray Hemitrygon akajei was described from 1,418 specimens, 682 males and 736 females, sampled year-round (2003–2014) from Ariake Bay to provide reproductive life history information for conservation and management of the species. Females reach sexual maturity at a larger size than males with the size at 50% sexual maturity 522.2 mm disc width (DW) and 321.5 mm DW, respectively. Male stingrays had semen in the seminal vesicles year-round. Dental sexual dimorphism was aseasonal. The mating period is protracted, spanning 7 consecutive months October–April but ovulation occurred during May. This suggests female sperm storage, which has not been described for dasyatid stingrays. Females have a single functional ovary and uterus (left) and reproduce via aplacental viviparity with lipid histotrophy. Gestation required 3 months with parturition during late July and early August. Uterine eggs without macroscopic embryos were observed during the first half of gestation suggesting a short period of arrested development or diapause. Developmental cohorts based on morphological features were described for embryos and can be used to characterize embryo growth and development for other stingray species. Observations of foetal mortality (1.25%) and morphologically abnormal embryos (0.72%) were uncommon. Pregnancy rate was 90% and reproduction was annual and synchronous. Hemitrygon akajei fecundity ranged from 7 to 25 and increased with female size. Although H. akajei is a medium-sized dasyatid ray, it has the highest fecundity reported for any batoid species. Elasmobranchs life histories usually are associated with a low degree of productivity that results in rapid population decline with increased fishing pressure. Producing high numbers of small young is a successful reproductive strategy for H. akajei that may be partly responsible for their resilience to fishing pressure and continued abundance in Ariake Bay. This study is the first to describe reproduction of H. akajei, an economically important top predator inhabiting coastal ecosystems throughout Asia.
Similar content being viewed by others
Introduction
Hemitrygon akajei is a whiptail stingray (Chondrichthyes: Dasyatidae) that inhabits sandy bottoms of temperate to tropical seas of the Northwest Pacific (Yamaguchi et al. 2013). Hemitrygon akajei is endemic to Asia, known from Japan, Korea, Taiwan, China, Thailand (east coast), Malaysia and Indonesia (Yano et al. 2005; Yamaguchi et al. 2013) and also from the Russian Federation, albeit with less frequency (Yamaguchi et al. 2013). In Japan, H. akajei is a common species found in coastal areas including brackish waters from Hokkaido to the Ogasawara Islands and Okinawa (Amaoka et al. 1989; Randall et al. 1997; Shinohara et al. 2005).
Ariake Bay, located in Kyushu, is one of the most productive bays in Japan (Takita and Yamaguchi 2009). Ariake Bay has high species diversity and a large resident population of elasmobranchs including five described and one undescribed Dasyatidae species (Furumitsu and Yamaguchi 2010). Elasmobranchs are non-target species caught by bottom trawlers targeting shrimps in summer and fishes in general during winter and comprise as much as 70–80% of the total catch (by mass) (Yamaguchi 2005). Hemitrygon akajei is the most common dasyatid caught by gill net (year-round), bottom trawl (May–August and November–February) and longline fisheries (year-round) (Yamaguchi 2009).
According to the IUCN Red List, landings of H. akajei are declining (Huveneers and Ishihara 2016). In 1972, the total catch of stingrays in Ariake Bay was 42 tons. Stingray landings increased to 310 tons in 1988, and subsequently declined to 113 tons in 2006, but no data concerning fishing effort during the same time frame is available for comparison (Kyushu Regional Agricultural Administration Office 1973–2007). A predator control program was implemented in 2001 to protect shellfish in Ariake Bay, and H. akajei was taken as part of that program; however, catch data are not available to determine the impact of the predator control program on H. akajei or other species (Yamaguchi 2009). Current landing records for H. akajei in Ariake Bay are not publicly available, preventing an assessment of changes in population size, distribution and trends over time.
Despite a commercial fishery and predator control program that target H. akajei, there are few biological studies of this species throughout its range and, consequently, little is known about its biology. The feeding habits of H. akajei from Tokyo Bay have been characterized (Taniuchi and Shimizu 1993). Dental sexual dimorphism, where males develop pointed cuspidate dentition to bite and hold females during copulation, is widespread among batoids and has been described for H. akajei from Tokyo Bay (Bigelow and Schroeder 1953; Taniuchi and Shimizu 1993; Nordell 1994; Kajiura and Tricas 1996). Temporal changes in tooth morphology associated with the mating season that have been observed for some dasyatid stingrays have not been investigated for H. akajei (Taniuchi and Shimizu 1993; Kajiura and Tricas 1996). Observations of mating behaviour and parturition for H. akajei were described from stingrays observed in aquaria (Hagiwara 1993).
There has been no study of the reproductive biology of H. akajei throughout its range, but some reproductive traits are common among dasyatids. Dasyatid stingrays have a single functional left ovary and uterus and reproduce via aplacental viviparity with lipid histotrophy (Capapé 1976; Snelson et al. 1988; Ribeiro et al. 2006; Veras et al. 2014). Mating is followed immediately by ovulation and fertilization with no evidence for female sperm storage. Gestation may include embryonic diapause, a temporary period of suspended or arrested embryonic development (Snelson et al. 1989; Morris 1999; Wyffels 2009; Waltrick et al. 2012). Maximum fecundity ranges from 1 for Hypanus marianae (Yokota and Lessa 2007) and Megatrygon microps (Nair and Soundararajan 1976; Pierce et al. 2008) to 13 for H. akajei (Hagiwara 1993) and Pteroplatytrygon violacea (Wilson and Beckett 1970). Dasyatid stingrays range from 30 cm (pale-edge sharpnose ray Telatrygon zugei) to more than 2 m (roughtail stingray Bathytoshia centroura) in disc width (DW) (Last et al. 2016). Fecundity increases with female size for select species, but this relationship cannot be generalized for all dasyatids.
Hemitrygon akajei is classified as “Near Threatened” (NT) by the IUCN Red List (Huveneers and Ishihara 2016). To accurately assess the current population status, impacts of fishing pressure and biological niche of H. akajei in Ariake Bay’s ecosystem, information about the life history and especially reproductive habits of H. akajei is needed. Reproductive periodicity, seasonality and variables such as size at maturity and fecundity are helpful to manage stocks and maintain a sustainable fishery. Given the lack of life history information for this species throughout its range and its value as a fisheries resource, the aim of this study was to characterize the reproductive biology of H. akajei from Ariake Bay including embryonic development and foetal mortality rate.
Materials and methods
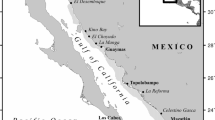
Study location and survey techniques. Ariake Bay is located in western Kyushu and has a gross area of 1,700 km2 (Fig. 1). The bay has extremely specific local environmental features including large tidal ranges (maximum 6 metres), fast currents and expansive tidal flats. The tidal flat area for this bay is the greatest in all of Japan with a gross area of 180–190 km2. The northern part of the bay is shallow with a depth less than 20 m, while the southern part of the bay is relatively deep. The mouth of the bay is the deepest area in the southern bay, almost 200 m deep and very narrow, forming a bottleneck barrier or pseudo-closed bay. Mixing with oceanic waters occurs at the mouth of the bay. Stingray specimens were sampled monthly April 2003–April 2014 from the catch of commercial fishing vessels operating in regional fisheries in Ariake Bay. Set nets were used in area A (3–8 m deep, an estuary), gill nets and set nets in area B (8–20 m deep), bottom trawlers in area C (35–65 m deep) and longlines in all three areas (3–200 m deep) (Fig. 1).
Morphometrics and reproductive data. Disc width (DW, nearest 0.5 mm), body mass (BM, nearest 0.001 g for embryos or 1 g for field specimens) and sex were recorded for each ray. The total number and number of mature males and females in the northern (A and B) and southern (C) areas were subjected to a Chi squared (χ2) test to determine if they differed from parity. For females, presence or absence of semen in the cloaca and cervix was noted and uterus mass (UM, nearest 0.1 g) was measured. For males, clasper length (CL, nearest 0.01 mm), the distance from the outer base of the clasper to its tip, was measured and the presence or absence of semen in the seminal vesicles was noted. Male maturity stages were determined based on the degree of development of testes and claspers: 1) immature, testes and claspers undeveloped; 2) premature or sub-adult, testes becoming lobular, vas deferens beginning to thicken and coil, clasper elongated and flexible, not calcified; and 3) mature, testes and seminal vesicle fully developed, vas deferens fully developed and coiled, semen may be present, claspers calcified. Female maturity stages were determined by the degree of development of the ovary and uterus: 1) immature, ovary comprising white, undifferentiated tissue, uterus thin; 2) premature or sub-adult, ovary carrying small, immature, opaque oocytes, uterus becoming thickened; and 3) mature, ovary containing ova with yolk, uterus fully developed and possibly with eggs or embryos (Furumitsu et al. 2010). For all males (n = 682), tooth shape was examined and described as pointed, transitional, or blunt. For females, the number of bite marks on the dorsal body surface where the underlying dermis was exposed was recorded (Kajiura et al. 2000). Changes in CL and UM relative to disc width were used as indicators of maturity (Yamaguchi and Kume 2009). Analysis of covariance (ANCOVA) was used to test for differences in the log-transformed linear DW–BM relationship between sexes. Pregnant females (n = 96) and females that were directly observed to abort one or more embryos upon landing (n = 25) were excluded from this analysis because of the high fecundity for this species.
Size at maturity. The percentage of mature individuals in relation to immature and premature individuals for each 10 mm size group was calculated and fitted to the logistic model, Y = [1 + e(aX + b)]−1, where Y is the proportion of individuals mature at size X, and a and b are empirical parameters (White and Dharmadi 2007; Kume et al. 2009). Parameters were derived using the statistical software KyPlot 5.0 (KyensLab Inc.). The size at which 50% of each sex attains sexual maturity (size at 50% sexual maturity) was calculated using the equation: size at 50% sexual maturity = − ba−1 (Kume et al. 2009).
Male and female reproductive status. The liver and gonad and associated epigonal organ mass (nearest 0.1 g) for mature males and females were collected to assess reproductive status. The gonadosomatic index (IG) was calculated using the formula: IG = [gonad mass/(body mass − gonad mass)] × 100. Specimens with compromised epigonal organs due to rapid decomposition were excluded from analysis. The monthly IG for mature males was used to approximate the mating season. Livers were removed and weighed to the nearest 0.1 g and the hepatosomatic index (IH) was calculated using the formula: IH = [liver mass/(body mass − liver mass)] × 100.
Testes removed from mature males were fixed in Bouin’s solution for 48 h and transferred to 70% ethanol. They were subsequently dehydrated and embedded in paraffin wax, sectioned to a thickness of 4–5 μm and stained with haematoxylin and eosin (HE). For the purpose of defining developmental stages of spermatogenesis, differentiating sperm cells were classified as spermatogonia (SG), spermatocytes (SC), spermatids (ST), immature spermatozoa (IS) and mature spermatozoa (MS) according to Conrath and Musick (2002). A representative cross section was selected from the middle of the right or left lobe and the testis classified into one of seven stages of spermatogenesis (I–VII) based on the most developed stage of sperm observed (Maruska et al. 1996). Monthly proportions of testis stage were calculated with and without the degenerate zone (VII) to characterize the stages of newly formed spermatocysts present in testis that are predominately degenerating.
For each mature female, the diameter of the 5 largest ovarian follicles was measured with an electronic calliper to the nearest 0.01 mm and averaged to assess ovulation. The mature uterus was bisected and the number of uterine eggs, mass of each egg and embryo DW (nearest 0.01 mm) was measured. Because Hemitrygon akajei was susceptible to capture-related uterine discharge, specimens that were observed to abort one or more embryos were removed from fecundity and uterine egg and embryo analyses. Uterine egg and embryo mass (nearest 0.001 g) and sex (when possible) were recorded. The number of male and female embryos for all gravid rays was subjected to a χ2 test to determine if the sex ratio differed from parity. The relationship between female DW and average uterine egg mass and female IH and fecundity were tested using linear regression. Size at birth was estimated from the largest embryos and the smallest free-swimming neonates. Embryos were classified into 9 developmental cohorts based on easily recognized morphological characteristics (Table 1). Using data extracted from the literature and including this study, a simple linear regression was used to model the relationships between DW of females and maximum size of young at birth and maximum fecundity for Dasyatinae species: Bathytoshia brevicaudata (Hagiwara 1993; Last and Stevens 2009), Bathytoshia centroura (Struhsaker 1969; Reed and Gilmore 1981; Capapé 1993), Bathytoshia lata (Uchida et al. 1990; Furumitsu et al. 2012), Dasyatis chrysonota (Ebert and Cowley 2009); Dasyatis hypostigma (Ribeiro et al. 2006), Dasyatis marmorata (Capapé and Zaouali 1995), Dasyatis pastinaca (Capapé 1976; Serena 2005; Saadaoui et al. 2015), Hemitrygon akajei (Hagiwara 1993), Hypanus americanus (Ramírez-Mosqueda et al. 2012; Tagliafico et al. 2013), Hypanus dipterurus (Smith et al. 2007), Hypanus guttatus (Yokota and Lessa 2006, 2007; Tagliafico et al. 2013), Hypanus longus (Villavicencio-Garayzar et al. 1994), Hypanus marianae (Yokota and Lessa 2007), Hypanus rudis (Springer and Collette 1971), Hypanus sabinus (Snelson et al. 1988; Johnson and Snelson 1996), Hypanus say (Snelson et al. 1989), Megatrygon microps (Nair and Soundararajan 1976; Pierce et al. 2008), Pteroplatytrygon violacea (Wilson and Beckett 1970; Mazzoleni and Schwingel 2002; Mollet et al. 2002; Hemida et al. 2003; Forselledo et al. 2008; Ribeiro-Prado and Amorim 2008; Veras et al. 2014), Taeniurops meyeni (White and Dharmadi 2007), Telatrygon biasa (White and Dharmadi 2007) and Telatrygon crozieri (Devadoss 1998; Raje and Zacharia 2009).
Results
Size distribution and sexual maturity. A total of 1,418 specimens, 682 males and 736 females, were collected from three sampling areas within Ariake Bay (Fig. 1). For all sample areas, males averaged [mean ± standard deviation (SD)] 256.0 ± 104.5 mm DW (range 105.0–466.0 mm) and 934.4 ± 946.2 g (range 43.5–4,009.9 g). For all sample areas, females averaged (mean ± SD) 368.5 ± 193.1 mm DW (range 109.5–900.0 mm) and 2,511.9 ± 3,344.7 g (range 42.2–25,100.0 g). The overall sex ratio (n = 1,418, F:M = 1:0.93) was not significantly different than 1 (χ2 = 2.06, d.f. = 1, P > 0.05); however, sex ratios did differ by sampling location within the bay. In the northern part of the bay (areas A and B) where it is relatively shallow and includes regions of brackish waters, females were more prevalent than males (F:M = 1:0.80, χ2 = 9.70, d.f. = 1, P < 0.05). In the southern part of the bay (area C) where the depth is 35–65 m, males were more prevalent than females although the difference was not significant (F:M = 0.90:1, χ2 = 1.86, d.f. = 1, P > 0.05). When the distribution is limited to mature specimens, females (n = 261) predominate in the northern part of the bay (areas A and B) (F:M = 1:0.64, χ2 = 15.42, d.f. = 1, P < 0.05), and males (n = 251) predominate in the southern part of the bay (area C) (F:M = 0.50:1, χ2 = 20.11, d.f. = 1, P < 0.05).
The size distributions for both sexes were bimodal (Fig. 2). The first mode represents immature male and female specimens of 150–199 mm DW and the second mode represents mature specimens, 350–399 mm DW for males and 550–599 mm DW for females (Fig. 2). In area A, an estuary, the number of small specimens (< 200 mm DW) was highest (68.7%) for both sexes. Large specimens (DW > 300 mm males, > 500 mm females) were frequent (82.0% and 67.4% for males and females, respectively) in the northern region (area B). The southern region (area C) has a similar distribution of males and females as the estuary with more immature females than mature females and a nearly equal number of immature and mature males. The smallest free-swimming specimens were 105.0 mm DW for males and 109.5 mm DW for females. The largest specimens were 466.0 mm DW and 4,009.9 g BM for males and 900.0 mm DW and 25,100.0 g BM for females. The largest female was twice the disc width and 6 times the mass of the largest male (Fig. 3). The relationship between DW and BM was not significantly different between males and females (F = 0.199, d.f. = 1, P > 0.05). The DW–BM model was BM = (2 × 10−5) DW3.126 (n = 1,289, r2 = 0.994). The smallest specimens that attained sexual maturity were 313 mm DW for males and 475 mm DW for females. All specimens larger than 347 mm DW for males and 560 mm DW for females were mature. Size at 50% sexual maturity (± 95% confidence interval) was 321.5 (321.4–321.5) mm DW for males and 522.2 (522.1–522.2) mm DW for females (Fig. 4a). Clasper length increased abruptly in rays > 300 mm DW (premature males, n = 25) with clasper growth slowing after the onset of sexual maturity (Fig. 4b). Uterus mass began to increase at approximately 500 mm DW, concomitant with the observation of premature females (n = 51), and continued to increase thereafter (Fig. 4c).
Hemitrygon akajei size at maturity for male and female rays samples from Ariake Bay, Japan from 2003 to 2014. (a) Size at 50% maturity was 321.5 mm disc width (DW) for males (n = 682, white circles) and 522.2 mm DW for females (n = 736, black circles); (b) Clasper length increased abruptly in rays > 300 mm DW (premature males, n = 25) and slowed after the onset of sexual maturity. (c) Uterus mass began to increase at approximately 500 mm DW for premature females (n = 51) and continued to increase thereafter
Testis and tooth morphology, spermatogenesis and temporal periodicity. The testes of the Hemitrygon akajei are paired dorsoventrally flattened organs located in the anterior peritoneal cavity and suspended from the dorsal wall by mesorchia. Both testes are functional. Development of seminiferous follicles proceeds radially from the central germinal zone in all directions towards the outer margin of the lobe where they connect to efferent ductules (Fig. 5). Follicles in the final stages of spermatogenesis are visible around the circumferential perimeter of each lobe (Fig. 5a-b).
Testis histology from mature Hemitrygon akajei collected from Ariake Bay, Japan. (a) Cross section through a single testicular lobe with germinal papilla (GP) and epigonal organ (EO) at the end of a 7 month protracted mating season with developing spermatocysts (stage I, II, III) and a degenerate zone (VII). (b) Cross section through a single testicular lobe at the beginning of a 7 month protracted mating period with all developing stages of spermatocysts (I–V) and mature sperm (VI); (c–d) stage I primary spermatogonia (SG); (e) stage II early spermatocysts with spermatogonial cells (SG); (f) stage III spermatocytes (SC) with sertoli cells (SE); (g) stage IV spermatids (ST); (h) stage V immature sperm (IS); (i) stage VI mature sperm (MS) and an empty spermatocyst (ES); (j) stage VII degenerate zone (DZ) and degenerate mature sperm (DMS)
All males had persisting degenerate zones from February to April (Fig. 5j, 6a), but newly formed stage II-III spermatocysts also were observed (Fig. 5e-f, 6b). Follicles containing unreleased and degenerate mature sperm were observed (Fig. 5j) and usually adjacent to follicles with viable mature sperm. Stage I males were observed in all months (Fig. 5c-d). Stage II (Fig. 5e) males occurred between January and June, peaking in February and reached stage IV (Fig 5g) in May and June. Stage V (Fig. 5h) was observed only in June. Stage VI (Fig. 5i) males were observed from June until January and all males collected between July and December had mature sperm in their testes. In January, approximately 70% of males were classified as stage VII with degenerate zones in their testes. Stage VII (Fig. 5j) specimens were observed from January through June. The percentage of males with semen in the seminal vesicles was 100% from September to May and decreased during June (57.6%) and July (56.5%) before rising again in August (92.6%). Immature males (n = 406) had blunt teeth, while premature males possessed transitional (n = 9) or blunt (n = 16) teeth. Tooth shape was not seasonal; teeth were pointed for all mature males (n = 251) throughout the year. Bite marks on the pectoral fins of mature females were observed every month except June, July and August.
Monthly hepatosomatic and gonadosomatic index and gonadal maturity stages for mature male Hemitrygon akajei collected from Ariake Bay, Japan, from 2003 to 2014. Each male was assigned a gonadal maturity stage based on the most developed stage of sperm observed from testicular histology considering (a) the most mature stage and (b) most mature stage disregarding the degenerate zone. Sample size for each month is indicated above the bar. Monthly distribution of the mean ± standard deviation for (c) gonadosomatic and (d) hepatosomatic index showed a clear seasonal variation, increasing from July, peaking during September and October, and decreasing from November
Reproductive cycle. Monthly IG of males (Fig. 6c) showed a clear seasonal variation, increasing from July, peaking during September and October, and decreasing from November. Monthly IH for males had a similar trend as IG, peaking in September (Fig. 6d). Monthly IG for females had clear seasonality, increasing from August, peaking in April and decreasing gradually from May through July (Fig. 7a). Monthly IH for females had a similar trend as IG, peaking in April concomitant with maximum ovarian follicle size (Fig. 7b-c). Average ovarian follicle diameter (mean ± SD) was smallest in September, 8.07 ± 1.30 mm (range 6.39–9.71 mm), and largest in April, 17.62 ± 2.96 mm (range 11.89–21.74 mm) (Fig. 7c). The largest ovarian follicle diameter observed in this study was 22.60 mm and the latest sampling date that large pre-ovulatory ova were observed was 30 May 2012. Semen was observed in the cloaca or cervix of 1 of 9 pre-ovulatory females in April. Semen was never observed in the cloaca or cervix of females with uterine eggs or embryos (April–August). Uterine eggs were observed for 1 of 10 mature females examined in 28 April and all mature females examined in May. From April to August, the monthly percentage of mature females (n = 185) that were gravid increased progressively from 10% in April to 79.1% in May peaking at 89.6% in June and 89.3% in July before declining abruptly to 7.1% in August. Beginning in September, semen was observed in the cloaca or cervix of females for 7 consecutive months.
Monthly hepatosomatic and gonadosomatic index and ovarian follicle diameter (mean ± standard deviation and sample size) for mature female Hemitrygon akajei collected from Ariake Bay, Japan, from 2003 to 2014. Peak (a) gonadosomatic index, (b) hepatosomatic index and (c) average diameter of the 5 largest ovarian follicles occurred in April before ovulation
Fecundity. Females with uterine eggs (n = 49) and embryos (n = 47) were collected from April to August. Fecundity ranged from 7 to 25 embryos per litter (n = 96, mean ± SD = 12.3 ± 3.5) and was positively correlated with female DW (linear regression; F = 23.37, d.f. = 95, P < 0.05) (Fig. 8a).
Hemitrygon akajei disc width and fecundity for females collected from Ariake Bay, Japan, from 2003 to 2014. (a) Fecundity ranged from 7 to 25 embryos per litter (n = 96, mean ± SD = 12.3 ± 3.5) and was positively correlated with female disc width (linear regression; F = 23.37, d.f. = 95, P < 0.05). (b) A uterine egg case (105.46 mm × 45.89 mm) containing 13 eggs (e)
Hemitrygon akajei uterine eggs were enclosed by a fragile translucent pale brown tertiary egg envelope (Fig. 8b) similar to other dasyatids (Wilson and Beckett 1970; Snelson et al. 1988, 1989; Capapé 1993; Capapé and Zaouali 1995; Johnson and Snelson 1996; Ebert and Cowley 2009). The uterine eggs of H. akajei were fragile, soft in texture, and pale to bright yellow in colour. Individual egg shape was variable and is constrained by the egg envelope and proximity to other eggs, but generally elongate and more planar than spherical. Invaginations in the yolk surface were obvious for eggs collected early in gestation and their prominence diminished with embryonic yolk consumption and sample fixation.
The mean combined mass for uterine eggs (mean ± SD) by female (n = 49) was 1.272 ± 0.271 (range 0.655–1.793 g). The mass varied widely between females with a significant relationship between DW and average uterine egg mass (linear regression; F = 4.45, d.f. = 48, P < 0.05) (Electronic Supplementary Material (ESM) Fig. S1a). There was not a significant relationship between the number of uterine eggs and their average mass (linear regression; F = 0.03, d.f. = 48, P > 0.05) (ESM Fig. S1b). Considering all females, the smallest egg by mass (0.544 g) was 25.5% of the largest egg (2.130 g) (ESM Fig. S1c). For most females, the range of uterine egg mass was small (ESM Fig. S2), but for four females the largest and smallest eggs differed by more than 0.5 g (Fig. 9). There was a significant relationship between female IH and fecundity (linear regression; r2 = 0.05, F = 5.05, P < 0.05).
The distribution of egg mass differences between the largest and smallest uterine egg by female (n = 49) for gravid Hemitrygon akajei collected from Ariake Bay, Japan, from 2003 to 2014. Within a litter, uterine eggs were provisioned with a similar mass of yolk to support initial embryo growth with the exception of four females who had at least one egg more than 0.5 g lighter than the largest egg
Embryonic development. Gestation for H. akajei in Ariake Bay requires approximately 3 months. For all eleven years combined, uterine eggs were observed from 28 April to 29 June and macroscopic embryos were present from 11 June to 11 August. Thirty-five gravid females collected during April and May (100%) and 22 of 29 gravid females collected in June (75.9%) had uterine eggs. The remaining 7 gravid females collected in June (24.1%) had uterine eggs with macroscopic embryos. Once embryos were observed, their development was rapid and complete within 1–1.5 months. The average [± standard error of the mean (SEM)] embryo size increased from 15.95 ± 12.20 mm DW in June to 118.28 ± 3.40 mm DW in August (Fig. 10). The earliest free-swimming specimen (113 mm DW and 59.4 g) was observed on 26 July and the latest embryos (114.88, 121.68 mm DW and 65.1, 77.1 g) were observed on 11 August. Both the largest embryo (128.84 mm DW and 85.1 g) and the smallest free-swimming specimen (105 mm DW and 44.3 g, male) were observed in August. Parturition occurred during late July through mid-August and size at birth was 105–130 mm DW.
Using previously published data for 19 dasyatid species and including this study, larger species bear larger young (linear regression; F = 68.67, d.f. = 27, P < 0.05) (Fig. 11a). The fecundity range for 20 dasyatids excluding H. akajei is 1–13 with most species less than 7 (Fig. 11b). There was no relationship between female maximum size and fecundity (Fig. 11b, linear regression; F = 0.012, d.f. = 36, P > 0.05).
A comparison of maximum size at birth and fecundity range for females from subfamily Dasyatinae. (a) Maximum size at birth and (b) fecundity range. Bathytoshia brevicaudata: 1 (Last and Stevens 2009) and 2 (Hagiwara 1993); Bathytoshia centroura: 3 (Capapé 1993), 4 (Struhsaker 1969) and 5 (Reed and Gilmore 1981); Bathytoshia lata: 6 (Uchida et al. 1990) and 7 (Furumitsu et al. 2012); Dasyatis chrysonota: 8 (Ebert and Cowley 2009); Dasyatis hypostigma: 9 (Ribeiro et al. 2006); Dasyatis marmorata: 10 (Capapé and Zaouali 1995); Dasyatis pastinaca: 11 (Capapé 1976), 12 (Serena 2005) and 13 (Saadaoui et al. 2015); Hemitrygon akajei: 14 (Hagiwara 1993); Hypanus americanus: 15 (Tagliafico et al. 2013) and 16 (Ramírez-Mosqueda et al. 2012); Hypanus dipterurus: 17 (Smith et al. 2007); Hypanus guttatus: 18 (Yokota and Lessa 2006, 2007) and 19 (Tagliafico et al. 2013); Hypanus longus: 20 (Villavicencio-Garayzar et al. 1994); Hypanus marianae: 21 (Yokota and Lessa 2007); Hypanus rudis: 22 (Springer and Collette 1971); Hypanus sabinus: 23 (Snelson et al. 1988) and 24 (Johnson and Snelson 1996); Hypanus say: 25 (Snelson et al. 1989); Megatrygon microps: 26 (Nair and Soundararajan 1976; Pierce et al. 2008); Pteroplatytrygon violacea: 27 (Wilson and Beckett 1970), 28 (Mollet et al. 2002), 29 (Hemida et al. 2003), 30 (Ribeiro-Prado and Amorim 2008), 31 (Mazzoleni and Schwingel 2002), 32 (Forselledo et al. 2008) and 33 (Veras et al. 2014); Taeniurops meyeni: 34 (White and Dharmadi 2007); Telatrygon biasa: 35 (White and Dharmadi 2007); Telatrygon crozieri: 36 (Raje and Zacharia 2009) and 37 (Devadoss 1998); 38 this study
A total of 623 uterine eggs without macroscopic embryos from 49 females and 559 embryos from 47 females were collected, sexed (n = 457) and assigned to developmental cohorts: stage 1 (n = 49), stage 2 (n = 3), stage 3 (n = 4), stage 4 (n = 3), stage 5 (n = 1), stage 6 (n = 7), stage 7 (n = 1), stage 8 (n = 21), and stage 9 (n = 7) based on their morphological characteristics (Table 1, Fig. 12). The sex ratio for embryos (F:M 1:0.90) was not significantly different from the expected 1:1 ratio (χ2 = 1.37, d.f. = 1, P > 0.05). As embryo DW increased, the mass of the external yolk sac decreased (Fig. 13). External yolk-sac mass was approximately 1 g for embryonic stages 2–3 and decreased to 0.065–0.461 g by stage 6. The yolk sac was mostly absorbed with a mass of less than 0.02 g at stage 8. Embryo body mass increased sharply after stage 6 (mean 5.459 g) and attained a mean of 61.479 g by stage 9. In conjunction with yolk depletion, embryonic nutrition transitioned to histotroph until parturition.
Embryonic development (Stages 1–9) of Hemitrygon akajei uterine embryos collected from Ariake Bay, Japan, from 2004 to 2014. (a) Stage 1: The egg is within an egg case and without a macroscopic embryo. (b) Stage 2: Macroscopic embryos are observed within an egg case (5.95 mm TL) (c) Stage 3: The pectoral fins do not exceed the posterior margin of the gills (6.47 mm DW) (d) Stage 4: The pectoral fins do not exceed the posterior margin of the eyes (15.19 mm DW) (e) Stage 5: The pectoral fins are anterior to the mouth but remain unfused (18.42 mm DW) (f) Stage 6: The pectoral fins are fused forming the disc, the yolk sac and gill filaments persist (34.60 mm DW) (g) Stage 7: Gill filaments are resorbed but the yolk sac remains (51.98 mm DW) (h) Stage 8: The yolk sac is resorbed and the body is free of pigmentation (86.00 mm DW) (i) Stage 9: Body pigmentation is present (119.59 mm DW). Scale bar for all images is 10 mm
Foetal mortality. A total of 559 embryos from 47 females were examined and, of them, 4 females had 5 dead embryos and 2 unfertilized or undeveloped eggs within the uterus (Fig. 14). The foetal mortality rate including all 47 gravid females was 1.25%. The first female (706 mm DW) with foetal mortality was collected on 13 July 2006. She had 16 embryos and 2 unfertilized or undeveloped eggs or 11.1% foetal mortality. The remaining 3 females (591 mm DW, 18 July 2006; 600 mm DW, 11 August 2006; 533 mm DW, 23 July 2012) had partially developed but dead embryos in their uteri resulting in foetal mortality rates of 13.3%, 14.3%, and 10.0%, by litter, respectively.
Undeveloped or unfertilized eggs or dead embryos found in the uteri of Hemitrygon akajei collected from Ariake Bay, Japan, from 2003 to 2014: (a) egg without embryonic development from a female collected in July 2006; (b-f) dead embryos from females collected in July 2007 (b-c), July 2012 (d) and August 2006 (e-f). Scale bar for all images is 10 mm
Abnormal embryos. Four morphologically abnormal, but live embryos alongside seven healthy embryos were observed in a single gravid female (521 mm DW) collected on 18 July 2007. All other females (n = 46) presented normal embryos. The embryos were at embryonic stage 8 and their abnormalities involved the head and pectoral fins (n = 2) or tail (n = 2) (Fig. 15). The embryos had significantly smaller disc widths compared to normal embryos at the same stage of development (one-way ANOVA; F = 6.65, d.f. = 10, P < 0.05). The percentage of morphologically abnormal H. akajei embryos observed in Ariake Bay was 0.72% (n = 4).
Discussion
Hemitrygon akajei is a top predator of coastal ecosystems throughout Asia that is targeted in regional fisheries. This is the first study of its reproductive life history throughout its range. All life stages were present year-round in Ariake Bay suggesting it is an essential habitat for the species. Reproduction of H. akajei is characterized by a protracted mating period, high fecundity, short gestation and a synchronous annual periodicity.
Size distribution and maturity.Hemitrygon akajei utilize different regions of Ariake Bay, dependent on their sex and maturity stage. Mature rays were segregated by sex and the smallest specimens were most abundant in the estuary areas. The bimodal size distribution for both males and females observed in Ariake Bay is similar to Atlantic stingray Hypanus sabinus in Florida Coastal Lagoons (Snelson et al. 1988). Snelson et al. (1988) attributed the bimodal distribution to be an artefact of sampling methodology, but partially based on the age, growth, and mortality characteristics of the population. For this study, the bimodal distribution may be related to habitat preference that accompanies changes in body size and sexual maturity as well as an artefact of sampling methodology. For example, shallower areas including estuaries are used often as a nursery ground and yield predominantly small immature specimens (Martins et al. 2018).
The maximum size for H. akajei females in Ariake Bay includes the largest on record for this species, exceeding the previously reported maximum size (app. 800 mm DW) from Tokyo Bay (Taniuchi and Shimizu 1993). However, the maximum size of males in Ariake Bay is approximately 10% or 50 mm smaller than that of Tokyo Bay (approximately 500 mm DW) (Taniuchi and Shimizu 1993). The size at maturity for H. akajei in Ariake Bay was smaller than described for the Tokyo Bay population, where all specimens > 400 mm DW for males and > 600 mm DW for females were mature (Taniuchi and Shimizu 1993). Geographical differences in size at maturity have been reported in starspotted smooth-hound Mustelus manazo from 5 populations around Japanese and Taiwanese waters (Yamaguchi et al. 2000), and cownose ray Rhinoptera bonasus from the Gulf of Mexico and Chesapeake Bay (Neer and Thompson 2005). In general, populations living in high latitude areas show a trend to attain a larger size, are slow to mature and have longer life spans and higher fecundity when compared to populations in low latitude areas for the same species (Cope 2006). Tokyo Bay and Ariake Bay are separated by 2° in latitude, with Tokyo Bay the higher of the two areas, which may account for the differences in ray size observed.
Reproduction. The reproductive cycle of male H. akajei indicates males are capable of reproduction year-round. Male IG mirrored IH similar to blue stingray Dasyatis chrysonota (Ebert and Cowley 2009) with peak IG following approximately four weeks after peak IH as observed for H. sabinus (Maruska et al. 1996). Spermatocycts containing mature testicular sperm dominate the testis at the same time as peak IG and IH, with recrudescence evident 3–4 months later. At the time of ovulation, semen for mating is provided from seminal vesicle stores because mature sperm are not observed in the testis.
Hemitrygon akajei females in Ariake Bay have an annual and synchronous reproductive cycle similar to H. sabinus and Hypanus say (Snelson et al. 1988, 1989; Maruska et al. 1996). Female IG and maximum follicle diameter followed a clear seasonal trend with a maximum in April corresponding to ovulation in May. The maximum ovarian follicle diameter (22.60 mm) was comparable to similar sized batoids (EMS Table S1). Female IH seasonality trends were less pronounced than IG, but peaked near the time of ovulation and were lowest during late gestation in common with H. sabinus (Maruska et al. 1996) and D. chrysonota (Ebert and Cowley 2009). Seasonal changes in IH for female lesser guitarfish Acroteriobatus annulatus were directly correlated with liver lipid content and decreased during vitellogenesis (Rossouw 1987). A continuous drop in female muscular and hepatic lipid content during gestation demonstrates the energy deficit females endure during gestation (Abdel-Aziz and El-Nady 1993). Hepatosomatic index is higher in H. akajei females compared to males and the range over the reproductive cycle is greater suggesting a larger investment and turnover of energy with reproductive output compared to males.
The pregnancy rate for mature females in Ariake Bay was 90% indicating a high rate of reproductive success. Only eight mature females sampled during summer were not gravid and five of the eight had below average body condition. Their IH fell within the lowest 10% observed for mature females. Poor condition may have been a limiting factor for reproductive success of these H. akajei females similar to observations for H. sabinus (Johnson and Snelson 1996).
Biting is an integral part of courtship and mating for elasmobranchs and changes in tooth morphology from a blunt molariform dentition to a pointed cuspidate dentition increase the male’s grip strength for holding females to facilitate copulation (Bigelow and Schroeder 1953; Kajiura and Tricas 1996). Females retain a blunt molariform dentition specialized for feeding year-round, but dental sexual dimorphism for males may be seasonal depending on species (Kajiura and Tricas 1996). The diet of male and female H. akajei in Tokyo Bay did not differ, but tooth morphology was described as pointed for mature males (Taniuchi and Shimizu 1993). However, seasonality for the dental dimorphism was not described. Once mature, H. akajei in Ariake Bay have pointed cuspidate dentition. Kajiura et al. (2000) reported wild caught H. sabinus females with recent mating-related bite marks healed after approximately two weeks in an aquarium. Assuming bite mark wound healing requires a similar time frame in H. akajei, the mating period of H. akajei in Ariake Bay extends maximally from September through May.
In addition to circumstantial evidence for mating from September through May from dermal bite marks, successful mating based on the presence of sperm in the female’s cloaca and/or cervix was confirmed for seven consecutive months, October through April. Because ovulation occurred during May, this sequela suggests female sperm storage, which has not been described for dasyatids or other rays in Ariake Bay. Both H. sabinus and H. akajei have a 7–8 month protracted mating period, but H. sabinus males have seasonally dimorphic dentition (Kajiura and Tricas 1996) while H. akajei males are permanently dimorphic. Further studies are needed to understand the implications of permanent versus seasonal dental dimorphism on feeding and reproduction for dasyatid stingrays. Why H. akajei and H. sabinus have a protracted mating period is not understood, but may be related to male competition, induced ovulation and androgen-influenced mating behavior (Maruska et al. 1996; Tricas et al. 2000).
For H. akajei, female maximum size was approximately twice that of males. Sexual dimorphism among chondrichthyans is associated with viviparity. For all species, the number of embryos is ultimately limited by uterine space, which in turn is determined by the size of the female (Martin and Cailliet 1988). Within species, larger females would be expected to have increased fecundity compared to smaller females to maximize reproductive fitness. Female size and fecundity were correlated for H. akajei, but this relationship cannot be generalized for all dasyatid stingrays. Considering this study, fecundity of 15 or more was observed for a wide range of H. akajei DW. Therefore, other factors such as body condition and IH may limit fecundity for females who have uterine capacity, but do not have resources for ova and embryo development.
Stingray fecundity is typically low and notoriously difficult to estimate for Myliobatiform rays, because embryos are commonly aborted due to capture-related stress (Smith et al. 2007; Adams et al. 2018). The average capture-induced parturition frequency for Dasyatinae from four studies on three species was 61.3% (Struhsaker 1969; Mollet 2002; Siqueira and Sant’Anna 2007; Saadaoui et al. 2015), whereas; in this study the frequency for H. akajei was 20.7%. Capture-induced parturition for moribund H. akajei females was observed commonly, but short gill net set time and immediate examination of specimens may have contributed to the decreased rate of capture-induced parturition observed in this study. A temporal effect on fecundity was reported for lobed stingaree Urolophus lobatus, where the mean number of embryos declined significantly from 2.9 to 1.3 during gestation, indicating some embryos were aborted during pregnancy (White et al. 2001) but no evidence for this was observed for H. akajei.
Fecundity for H. akajei in Ariake Bay is highest among reported values for this species across Asia and the highest for aplacental viviparous rays with a single functional uterus and histotrophy (Hagiwara 1993; Kim et al. 2005). Hemitrygon akajei’s high fecundity is both interesting and important to consider when evaluating their reproductive strategy and abundance in Ariake Bay. Considering the fecundity of three species with a similar maximum size as H. akajei: D. chrysonota (Ebert and Cowley 2009), Hypanus dipterurus (Smith et al. 2007) and H. say (Snelson et al. 1989), the number of embryos for H. akajei is 3.6–8.3 fold greater. Additionally, comparing stingray size at parturition for the same three species, (D. chrysonota, 172–184 mm DW, H. dipterurus, 190–280 mm DW, and H. say, 150–170 mm DW), size at birth for H. akajei is smallest (105–130 mm DW). Hemitrygon akajei utilizes a reproductive strategy that yields a high number of smaller young rather than a small number of large young. This direct trade-off between number of embryos and the size at birth of each embryo has been reported for other viviparous shark species including hemigaleids, carcharhinids and sphyrnids (Compagno 1988). The implications of this trade-off include higher predation and lower survival rate compared to species that produce few but larger young, although data from this study cannot address survivorship directly.
Embryonic growth and development. Gestation for H. akajei in Ariake Bay requires approximately three months, but uterine eggs without macroscopic embryos were observed during the first half of the gestation period suggesting that H. akajei utilizes diapause, a period of arrested development, observed for other elasmobranchs (Wyffels 2009). Once macroscopic embryos were observed, the time required for H. akajei to complete embryonic development (1–1.5 months) was similar to H. say excluding diapause [1.5–2 months, (Snelson et al. 1989)] and H. sabinus which does not have diapause [approximately two months, (Snelson et al. 1988)]. Gestation time for D. chrysonota [2–3 months, (Ebert and Cowley 2009)] and Dasyatis marmorata [three months, (Capapé and Zaouali 1995)] is almost twice as long as that of H. akajei. Embryonic nutrition can be derived from yolk and histotroph until midgestation (stage 6) when the external yolk sac is re-absorbed and yolk exhausted. Concomitantly, the uterine mucosa develops trophonemata that provide surface area for gas exchange and histotroph to nourish the embryos for the latter half of development until parturition (Hamlett et al. 1985). There are few studies on detailed developmental processes of dasyatids that include photos of embryonic stages (Forselledo et al. 2008; Ebert and Cowley 2009), and thus this study provides very important and fundamental information to characterize embryonic development and the transition of nutrient source during gestation for H. akajei and stingrays in general.
This is the first report for size at birth for wild specimens of H. akajei. The size at birth, estimated in this study, 105–130 mm DW and 44.3–85.1 g, is considerably smaller than neonates, 193–195 mm DW, 330–390 g, from females at Shimoda Floating Aquarium, Shizuoka Pref., Japan (Hagiwara 1993). A similar observation was reported for spotted eagle rays Aetobatus narinari, where size at birth for aquarium specimens (400–590 mm DW) was larger than that of wild specimens (170–360 mm DW) (Mahon et al. 2004), but no difference was observed between Hypanus americanus aquarium specimens (200–340 mm DW; Henningsen 2000) and wild specimens (230–340 mm DW; Ramírez-Mosqueda et al. 2012). The reported size at birth differences between wild and aquarium rays may be caused by delayed parturition, optimal feeding or reduced fecundity (Janse et al. 2010).
This study is the first report of foetal mortality rate for wild-caught viviparous rays. Baremore and Hale (2012) reported 34% of Carcharhinus plumbeus gravid females examined (n = 99) carried one or more unfertilized eggs (mean 1.3). In addition to unfertilized eggs, three mummified embryos were observed, two within one uterus. They concluded that the foetal mortality rate (unfertilized uterine eggs and mummified embryos) for C. plumbeus is relatively low and the phenomenon of embryo mummification and occurrence of unfertilized ova is common among shark species. There are additional reports on the occurrence of mummified embryos for Carcharhinus falciformis (Sandoval-Castillo and Villavicencio-Garayzar 2008), Carcharhinus obscurus (Clark and von Schmidt 1965), C. plumbeus (Clark and von Schmidt 1965; Rosa-Molinar et al. 1983; Baremore and Hale 2012), Galeocerdo cuvier (Clark and von Schmidt 1965), Mustelus norrisi (Clark and von Schmidt 1965) and Triaenodon obesus (Randall 1977). These species are all viviparous with a yolk-sac placenta except G. cuvier which is aplacental viviparous.
In general, foetal mortality is higher for oviparous species when compared with viviparous species. Six Chiloscyllium punctatum females laid a total of 692 eggs over two years, but 67.1% were not viable, 11.6% of embryos died during incubation and 21.4% yielded hatchlings (Harahush et al. 2007). In other reports for oviparous elasmobranchs, the hatching rate was 27.1% for Chiloscyllium plagiosum (Chen and Liu 2006), 73.0% for Dipturus laevis, 59.4% for Leucoraja ocellata and 37.5% for Amblyraja radiata (Parent et al. 2008). Palm et al. (2011) suggested that the average viability rate for A. radiata (74.1%) is one of the highest recorded in the primary literature for any skate species. However, these observations for oviparous species are from fish in aquariums and a direct comparison with wild and viviparous species such as H. akajei is not valid. Regardless of reproductive mode, 98.75% viability as observed in this study for H. akajei is high among elasmobranchs.
Adult specimens with abnormal morphology are observed only rarely in batoid species, but abnormal embryos do occur, as described in this study. Springer (1960) estimated that the frequency of observing a litter with one or more abnormal specimens of C. plumbeus is one in 500 to 1,000 normal litters. Records on the occurrence of abnormal specimens including embryos in batoid species include a 1.3% occurrence rate from total catches of Pteroplatytrygon violacea (Ribeiro-Prado et al. 2008) and 0.31% for Urotrygon rogersi (Mejía-Falla et al. 2011).
Free-swimming H. akajei females with morphological abnormalities were sampled off the coast of Nezugaseki Village, Yamagata Prefecture, Japan, in 1970 (152 mm DW, 130 g BM; Honma and Sugihara 1971) and Ise Bay, Mie Prefecture, Japan, in 1994 (115 mm DW; Yamaguchi 2004). Other batoids where free-swimming females with morphological abnormalities have been sampled include H. dipterurus caught off the coast of Las Losas, Antofagasta, Chile, in 1992 (216 mm DW; Lamilla et al. 1995) and the Gulf of California (Estero del Soldado, Sonora, Mexico) (364 mm DW, 1,130 g BM; Blanco-Parra and Niño-Torres 2011) and Hypanus longus from the southern Gulf of California, Mexico (367 mm DW, 859 g BM; Escobar-Sánchez et al. 2009). Abnormal specimens described above, except H. dipterurus from the Gulf of California, were collected shortly after birth as estimated from reported DW range at birth in this or previous studies (Hagiwara 1993; Villavicencio-Garayzar et al. 1994; Smith et al. 2007). The H. dipterurus caught off Sonora, in the Gulf of California, Mexico, was 364 mm DW, much larger than the reported range for size at birth (190–280 mm DW; Smith et al. 2007) but still not mature (Blanco-Parra and Niño-Torres 2011). For all rays, the physical deformity observed was associated with the pectoral fins (Honma and Sugihara 1971; Lamilla et al. 1995; Yamaguchi 2004; Escobar-Sánchez et al. 2009; Blanco-Parra and Niño-Torres 2011). As a result, for rays that survive to parturition, motility and feeding might be affected adversely resulting in high mortality rate (Yamaguchi 2004; Escobar-Sánchez et al. 2009). Mature females with morphological abnormalities have been observed for only one ray, P. violacea, 475 mm DW, which was gravid at the time of collection with an embryo that was morphologically abnormal as well (Ribeiro-Prado et al. 2008). Species in which mature male specimens with morphological abnormalities were sampled include H. akajei (390 mm DW, 2,400 g BM) and Hypanus guttatus (520 mm DW, 5,000 g BM) caught off Maizuru in the Sea of Japan (Yamaguchi 2004) and the northern coast of Colombia (Ramírez-Hernandez et al. 2011), respectively. All morphologically abnormal mature rays had incompletely fused pectoral fins. Among 1,418 specimens of H. akajei spanning 11 years from Ariake Bay, no abnormal specimens (except embryos) were observed. The frequency of abnormal embryonic specimens (0.72%) of H. akajei in Ariake Bay is relatively low.
Conservation and future study.Hemitrygon akajei is a target species of longline fisheries in coastal areas including estuaries such as Ariake Bay but catch data is not available to assess population structure and monitor the effects of regional fisheries over time. Because fishermen land fish greater than 500 mm DW, and release fish smaller than 500 mm DW, fishing pressure is biased towards mature females. Removing the largest females impacts the next generation more than smaller mature females, because fecundity increases with female size. This has important implications for conservation and management practices. The high fecundity of this elasmobranch species likely contributes towards sustaining its biomass and resilience to fishing pressure (Kindsvater et al. 2016). Confirmation of embryonic diapause and its role in the reproductive strategy is the subject of current research efforts in the laboratory. Understanding how the unusually high fecundity of this elasmobranch contributes to its resilience is important for successful management of the species.
References
Abdel-Aziz SH, El-Nady FS (1993) Lipid dynamics in the common torpedo, Torpedo torpedo, from the south eastern Mediterranean. J Fish Biol 43:155–162
Adams KR, Fetterplace LC, Davis AR, Taylor MD, Knott NA (2018) Sharks, rays and abortion: The prevalence of capture-induced parturition in elasmobranchs. Biol Conserv 217:11–27
Amaoka K, Nakaya K, Yabe M (1989) Fishes of Usujiri and adjacent waters in southern Hokkaido, Japan. Bull Fac Fish Hokkaido Univ 40:254–277
Baremore IE, Hale LF (2012) Reproduction of the sandbar shark in the western North Atlantic Ocean and Gulf of Mexico. Mar Coast Fish: Dyn Manag Ecosyst Sci 4:560–572
Bigelow HB, Schroeder WC (1953) Fishes of the Western North Atlantic, Part 2. Sawfishes, Guitarfishes, Skates and Rays; Chimaeroids. Sears Foundation for Marine Research, Yale University, New Haven
Blanco-Parra MdP, Niño-Torres CA (2011) Morphological abnormality in a diamond stingray, Dasyatis dipterura (Jordan & Gilbert, 1880) (Chondrichthyes: Dasyatidae), from the Gulf of California, Mexico. Cah Biol Mar 52:357–360
Capapé C (1976) Contribution à la biologie des Dasyatidae des côtes tunisiennes. I. Dasyatis pastinaca (Linne, 1758). Répartition géographique et bathymétrique, sexualité, reproduction, fécondité. Ann Mus Civ Stor Nat Giacomo Doria 81:22–32
Capapé C (1993) New data on the reproductive biology of the thorny stingray, Dasyatis centroura (Pisces: Dasyatidae) from off the Tunisian coasts. Environ Biol Fish 38:73–80
Capapé C, Zaouali J (1995) Reproductive biology of the marbled stingray, Dasyatis marmorata (Steindachner, 1892) (Pisces: Dasyatidae) in Tunisian waters (central Mediterranean). J Aquaricult Aquat Sci 7:108–119
Chen W-K, Liu K-M (2006) Reproductive biology of whitespotted bamboo shark Chiloscyllium plagiosum in northern waters off Taiwan. Fish Sci 72:1215–1224
Clark E, von Schmidt K (1965) Sharks of the central Gulf Coast of Florida. Bull Mar Sci 15:13–83
Compagno LJV (1988) Sharks of the order Carcharhiniformes. Princeton University Press, Princeton, New Jersey
Conrath CL, Musick JA (2002) Reproductive biology of the smooth dogfish, Mustelus canis, in the northwest Atlantic Ocean. Environ Biol Fish 64:367–377
Cope JM (2006) Exploring intraspecific life history patterns in sharks. Fish Bull 104:311–320
Devadoss P (1998) Observations on the breeding and development in some batoid fishes. Indian J Fish 45:271–283
Ebert DA, Cowley PD (2009) Reproduction and embryonic development of the blue stingray, Dasyatis chrysonota, in southern African waters. J Mar Biol Assoc UK 89:809–815
Escobar-Sánchez O, Galván-Magaña F, Downton-Hoffmann CA, Carrera-Fernández M, Alatorre-Ramírez VG (2009) First record of a morphological abnormality in the longtail stingray Dasyatis longa (Myliobatiformes: Dasyatidae) in the Gulf of California, Mexico. Mar Biodivers Rec 2:1–3
Forselledo R, Pons M, Miller P, Domingo A (2008) Distribution and population structure of the pelagic stingray, Pteroplatytrygon violacea (Dasyatidae), in the south-western Atlantic. Aquat Living Resour 21:357–363
Furumitsu K, Yamaguchi A (2010) Proposal of a new Japanese name for Dasyatis sp. Bull Fac Fish Nagasaki Univ 91:61–63
Furumitsu K, Yamaguchi Y, Prasert T, Horinouchi M, Yamaguchi A (2012) The cow stingray, Dasyatis cf. ushiei from the Andaman Sea, Trang, Thailand, and off Kuroshima, Nagasaki, Japan. Rep Japan Soc Elasmobranch Stud 48:1–5
Furumitsu K, Zhang J, Yamaguchi A (2010) Redescription of a poorly known stingray, Dasyatis laevigata (Chondrichthyes: Dasyatidae), with notes on some biological aspects in Ariake Sea, Japan. Species Divers 15:139–154
Hagiwara S (1993) Keeping and reproduction of Chondrichthyans in captivity at Shimoda Floating Aquarium. Rep Japan Soc Elasmobranch Stud 30:1–18
Hamlett WC, Wourms JP, Smith JW (1985) Stingray placental analogues: structure of trophonemata in Rhinoptera bonasus. J Submicrosc Cytol 17:541–550
Harahush BK, Fischer ABP, Collin SP (2007) Captive breeding and embryonic development of Chiloscyllium punctatum Muller & Henle, 1838 (Elasmobranchii: Hemiscyllidae). J Fish Biol 71:1007–1022
Hemida F, Seridji R, Ennajar S, Bradaϊ MN, Collier E, Guélorget O, Capapé C (2003) New observations on the reproductive biology of the pelagic stingray, Dasyatis violacea Bonaparte, 1832 (Chondrichthyes: Dasyatidae) from the Mediterranean Sea. Acta Adriat 44:193–204
Henningsen AD (2000) Notes on reproduction in the routhern rtingray, Dasyatis americana (Chondrichthyes: Dasyatidae), in a captive environment. Copeia 2000:826–828
Honma Y, Sugihara C (1971) A stingray, Dasyatis akajei, with aberrant pectoral fins from the Japan Sea. Jpn J Ichthyol 18:187–189
Huveneers C, Ishihara H (2016) Hemitrygon akajei. IUCN red list of threatened species 2016. http://dx.doi.org/10.2305/IUCN.UK.2016-3.RLTS.T60148A104113240.en. Accessed 23 April 2018
Janse M, Verbeek M, Wennekers F, Hendriks R, te Vruchte T, Dogger R, Arentz B, Rozier A, Kolkman R (2010) Notes on captive breeding of white spotted eagle ray (Aetobatus narinari) with specific emphasis on the parturition. Drum and Croaker 41:3–7
Johnson MR, Snelson FF Jr (1996) Reproductive life history of the Atlantic stingray, Dasyatis sabina (Pisces, Dasyatidae), in the freshwater St. Johns River, Florida. Bull Mar Sci 59:74–88
Kajiura SM, Sebastian AP, Tricas TC (2000) Dermal bite wounds as indicators of reproductive seasonality and behaviour in the Atlantic stingray, Dasyatis sabina. Environ Biol Fish 58:23–31
Kajiura SM, Tricas TC (1996) Seasonal dynamics of dental sexual dimorphism in the Atlantic stingray Dasyatis sabina. J Exp Biol 199:2297–2306
Kim I-S, Choi Y, Lee C-L, Lee Y-J, Kim B-J, Kim J-H (2005) Family Dasyatidae. In: Kim I-S, Choi Y, Lee C-L, Lee Y-J, Kim B-J, Kim J-H (eds) Illustrated Book of Korean Fishes. Kyohak Publishing, Seoul, Korea, pp 74–76
Kindsvater HK, Mangel M, Reynolds JD, Dulvy NK (2016) Ten principles from evolutionary ecology essential for effective marine conservation. Ecology and Evolution 6:2125–2138
Kume G, Furumitsu K, Tanaka S, Yamaguchi A (2009) Reproductive biology of the guitarfish Rhinobatos hynnicephalus (Batoidea: Rhinobatidae) in Ariake Bay, Japan. Environ Biol Fish 85:289–298
Kyushu Regional Agricultural Administration Office (1973-2007) Annual Report of Statistics on Agriculture, Forestry and Fisheries. Ministry of Agriculture Forestry and Fisheries of Japan, Tokyo
Lamilla JG, Pequeño GR, Kong IU (1995) Dasyatis brevis (Garman, 1880) segunda especie de Dasyatidae registrada para Chile (Chondrichthyes, Myliobatiformes). Estud Oceanol 14:23–27
Last PR, Manjaji-Matsumoto BM, Naylor GJP, White WT (2016) Family Dasyatidae. In: Last PR, White WT, de Carvalho MR, Séret B, Stehmann MFW, Naylor GJP (eds) Rays of the World, First. Cornell University Press, Ithaca, New York, pp 522–618
Last PR, Stevens JD (2009) Family Dasyatidae. In: Last PR, Stevens JD (eds) Sharks and Rays of Australia, Second Edition. CSIRO Publishing, Melbourne, Australia, pp 429–462
Mahon J, Chua F, Newman P (2004) Successful spotted eagle ray (Aetobatus narinari) breeding program and details of an assisted birth. In: Mohan P, Smith M (eds) DRUM and CROAKER Special Edition No 2. Ohio Biological Survey, Columbus, Ohio, pp 104–107
Martin LK, Cailliet GM (1988) Aspects of the reproduction of the bat ray, Myliobatis californica, in central California. Copeia 1988:754–762
Martins APB, Heupel MR, Chin A, Simpfendorfer CA (2018) Batoid nurseries: definition, use and importance. Mar Ecol Prog Ser 595:253–267
Maruska KP, Cowie EG, Tricas TC (1996) Periodic gonadal activity and protracted mating in elasmobranch fishes. J Exp Zool 276:219–232
Mazzoleni RC, Schwingel PR (2002) Aspectos da biologia das espécies capturadas por espinhel pelágico na região sul das ilhas de Trindade e Martin Vaz no verão de 2001. Notas Téc FACIMAR 6:51–57
Mejía-Falla PA, Navia AF, Muñoz LA (2011) First record of morphological abnormality in embryos of Urotrygon rogersi (Jordan & Starks, 1895) (Myliobatiformes: Urotrygonidae) in the tropical Eastern Pacific. Lat Am J Aquat Res 39:184–188
Mollet HF (2002) Distribution of the pelagic stingray, Dasyatis violacea (Bonaparte, 1832), off California, Central America, and worldwide. Mar Freshw Res 53:525–530
Mollet HF, Ezcurra JM, O’Sullivan JB (2002) Captive biology of the pelagic stingray, Dasyatis violacea (Bonaparte, 1832). Mar Freshw Res 53:531–541
Morris JA (1999) Aspects of the reproductive biology of the bluntnose stingray, Dasyatis say, in the Indian River lagoon system. Masters Thesis, University of Central Florida
Nair RV, Soundararajan R (1976) On the occurrence of the sting ray Dasyatis (Dasyatis) microps (Annandale) on the Madras Coast and in the Gulf of Mannar. Indian J Fish 23:273–277
Neer JA, Thompson BA (2005) Life history of the cownose ray, Rhinoptera bonasus, in the northern Gulf of Mexico, with comments on geographic variability in life history traits. Environ Biol Fish 73:321–331
Nordell SE (1994) Observations of the mating behavior and dentition of the round stingray, Urolophus halleri. Environ Biol Fish 39:219–229
Palm BD, Koester DM, Driggers III WB, Sulikowski JA (2011) Seasonal variation in fecundity, egg case viability, gestation, and neonate size for little skates, Leucoraja erinacea, in the Gulf of Maine. Environ Biol Fish 92:585–589
Parent S, Pépin S, Genet J-P, Misserey L, Rojas S (2008) Captive breeding of the barndoor skate (Dipturus laevis) at the Montreal Biodome, with comparison notes on two other captive-bred skate species. Zoo Biol 27:145–53
Pierce SJ, White WT, Marshall AD (2008) New record of the smalleye stingray, Dasyatis microps (Myliobatiformes: Dasyatidae), from the western Indian Ocean. Zootaxa 1734:65–68
Raje SG, Zacharia PU (2009) Investigations on fishery and biology of nine species of rays in Mumbai waters. Indian J Fish 56:95–101
Ramírez-Hernandez A, Paiacios-Barreto P, Gaitán-Espitia JD, Reyes F, Ramírez J (2011) Morphological abnormality in the longnose stingray Dasyatis guttata (Myliobatiformes: Dasyatidae) in the Colombian Caribbean. Cybium 35:79–80
Ramírez-Mosqueda E, Pérez-Jiménez JC, Mendoza-Carranza M (2012) Reproductive parameters of the southern stingray Dasyatis americana in southern gulf of Mexico. Lat Am J Aquat Res 40:335–344
Randall JE (1977) Contribution to the biology of the whitetip reef shark (Triaenodon obesus). Pac Sci 31:143–164
Randall JE, Ida H, Kato K, Pyle RL, Earle JL (1997) Annotated checklist of the inshore fishes of the Ogasawara Islands. Natl Sci Mus Monogr 11:1–74, 19 plates
Reed JK, Gilmore RG (1981) Inshore occurrence and nuptial behavior of the roughtail stingray, Dasyatis centroura (Dasyatidae), on the continental shelf, east central Florida. Northeast Gulf Sci 5:59–62
Ribeiro-Prado CC, Amorim AF (2008) Fishery biology on pelagic stingray Pteroplatytrygon violacea caught off southern Brazil by longliners settled in São Paulo State (2006-2007). Collect Vol Sci Pap ICCAT 62:1883–1891
Ribeiro-Prado CC, Oddone MC, Gonzalez MMB, Amorim AFd, Capapé C (2008) Morphological abnormalities in skates and rays (Chondrichthyes) from off southeastern Brazil. Arq Ciên Mar, Fortaleza 41:21–28
Ribeiro L, Rodrigues G, Nunan GW (2006) First record of a pregnant female of Dasyatis hypostigma, with description of the embryos. Environ Biol Fish 75:219–221
Rosa-Molinar E, Williams CS, Collard S (1983) Fetal mummification in the sandbar shark, Carcharhinus plumbeus (Nardo, 1827). J Wildl Dis 19:156–158
Rossouw GJ (1987) Function of the liver and hepatic lipids of the lesser sand shark, Rhinobatos annulatus (Müller & Henle). Comp Biochem Physiol B Comp Biochem 86:785–790
Saadaoui A, Saidi B, Enajjar S, Bradai MN (2015) Reproductive biology of the common stingray Dasyatis pastinaca (Linnaeus, 1758) off the Gulf of Gabès (Central Mediterranean Sea). Cah Biol Mar 56:389–396
Sandoval-Castillo J, Villavicencio-Garayzar C (2008) Fetal mummification in silky shark (Carcharhinus falciformis) from the Gulf of California, Mexico. Braz Arch Biol Technol 51:551–554
Serena F (2005) Dasyatidae. In: Serena F (ed) FAO species identification guide for fishery purposes. Field identification guide to the sharks and rays of the Mediterranean and Black Sea. Food and Agriculture Organisation of the United Nations, Rome, pp 67–70
Shinohara G, Sato T, Aonuma Y, Horikawa H, Matsuura K, Nakabo T, Sato K (2005) Annotated checklist of deep-sea fishes from the waters around the Ryukyu Islands, Japan. Natl Sci Mus Monogr 29:385–452
Siqueira AEd, Sant’Anna VBd (2007) Data on the pelagic stingray, Pteroplatytrygon violacea (Bonaparte, 1832) (Myliobatiformes: Dasyatidae) caught in the Rio de Janeiro coast. Braz J Oceanogr 55:323–325
Smith WD, Cailliet GM, Melendez EM (2007) Maturity and growth characteristics of a commercially exploited stingray, Dasyatis dipterura. Mar Freshw Res 58:54–66
Snelson FF Jr, Williams-Hooper SE, Schmid TH (1988) Reproduction and ecology of the Atlantic stingray, Dasyatis sabina, in Florida coastal lagoons. Copeia 1988:729–739
Snelson FF Jr, Williams-Hooper SE, Schmid TH (1989) Biology of the bluntnose stingray, Dasyatis sayi, in Florida coastal lagoons. Bull Mar Sci 45:15–25
Springer S (1960) Natural history of the sandbar shark, Eulamia milberti. Fish Bull 61:1-38
Springer S, Collette BB (1971) The Gulf of Guinea stingray, Dasyatis rudis. Copeia 1971:338–341
Struhsaker P (1969) Observations on the biology and distribution of the thorny stingray, Dasyatis centroura (Pisces: Dasyatidae). Bull Mar Sci 19:456–481
Tagliafico A, Rago N, Salomé Rangel M (2013) Aspectos biológicos de las rayas Dasyatis guttata y Dasyatis americana (Myliobatiformes: Dasyatidae) capturadas por la pesquería artesanal de la Isla de Margarita, Venezuela. Rev Biol Mar Oceanogr 48:365–373
Takita T, Yamaguchi A (2009) Fishes in estuarine and tidal flat ecosystems. Status of endangered fishes in Ariake Sound. Tokai University Press, Kanagawa, Japan
Taniuchi T, Shimizu M (1993) Dental sexual dimorphism and food habits in the stingray Dasyatis akajei from Tokyo Bay, Japan. Nippon Suisan Gakkaishi 59:53–60
Tricas TC, Maruska KP, Rasmussen LEL (2000) Annual cycles of steroid hormone production, gonad development, and reproductive behavior in the Atlantic stingray. Gen Comp Endocrinol 118:209–225
Uchida S, Toda M, Kamei Y (1990) Reproduction of elasmobranchs in captivity. In: Pratt JHL, Gruber SH, Taniuchi T (eds) Elasmobranchs as living resources: advances in the biology, ecology, systematics, and the status of the fisheries. NOAA Technical Report NMFS 90. U.S. Department of Commerce, National Oceanic and Atmospheric Administration, National Marine Fisheries Service, Seattle, Washington, pp 211–237
Veras DP, Hazin FHV, Branco ISL, Tolotti MT, Burgess GH (2014) Reproductive biology of the pelagic stingray, Pteroplatytrygon violacea (Bonaparte, 1832), in the equatorial and south-western Atlantic Ocean. Mar Freshw Res 65:1035–1044
Villavicencio-Garayzar CJ, Hoffmann CD, Melendez EM (1994) Tamaño y reproducción de la raya Dasyatis longus (Pisces: Dasyatidae), en Bahía Almejas, Baja California Sur, México. Rev Biol Trop 42:375–377
Waltrick D, Awruch C, Simpfendorfer C (2012) Embryonic diapause in the elasmobranchs. Rev Fish Biol Fish 22:849–859
White WT, Dharmadi (2007) Species and size compositions and reproductive biology of rays (Chondrichthyes, Batoidea) caught in target and non-target fisheries in eastern Indonesia. J Fish Biol 70:1809–1837
White WT, Platell ME, Potter IC (2001) Relationship between reproductive biology and age composition and growth in Urolophus lobatus (Batoidea: Urolophidae). Mar Biol 138:135–147
Wilson PC, Beckett JS (1970) Atlantic Ocean distribution of the pelagic stingray, Dasyatis violacea. Copeia 1970:696–707
Wyffels JT (2009) Embryonic development of chondrichthyan fishes—a review. In: Kunz YW, Luer CA, Kapoor BG (eds) Development of non-teleost fishes. Science Publishers, Enfield, New Hampshire, pp 1–103
Yamaguchi A (2004) Abnormal specimens of Dasyatis akajei from Japan Sea, Myliobatis tobijei and Aetobatus fragellum from Ariake Sound. Rep Japan Soc Elasmobranch Stud 40:44–45
Yamaguchi A (2005) On the yearly catch variation of rays in Ariake Sound. Rep Japan Soc Elasmobranch Stud 41:8–12
Yamaguchi A (2009) Sharks and rays in Ariake Sound. In: Takita T, Yamaguchi A (eds) Fishes in estuarine and tidal flat ecosystems: Status of endangered fishes in Ariake Sound. Tokai University Press, Kanagawa, Japan, pp 33–64
Yamaguchi A, Aonuma Y, Yagishita N, Yoshino T (2013) Dasyatidae. In: Nakabo T (ed) Fishes of Japan with pictorial keys to the species Third edition. Tokai University Press, Kanagawa, Japan, pp 220–226
Yamaguchi A, Kume G (2009) Reproductive biology of the fanray, Platyrhina sinensis (Batoidea: Platyrhinidae) in Ariake Bay, Japan. Ichthyol Res 56:133–139
Yamaguchi A, Taniuchi T, Shimizu M (2000) Geographic variations in reproductive parameters of the starspotted dogfish, Mustelus manazo, from five localities in Japan and in Taiwan. Environ Biol Fish 57:221–233
Yano K, Ali A, Gambang AC, Hamid IA, Razak SA, Zainal A (2005) Dasyatidae. In: Yano K, Ali A, Gambang AC, Hamid IA, Razak SA, Zainal A (eds) Sharks and rays of Malaysia and Brunei Darussalam. Marine Fishery Ressources Development and Management Department (MFRDMD), Southeast Asian Fisheries Developement Center (SEAFDEC), Terengganu, Malaysia, pp 361–455
Yokota L, Lessa RP (2006) A nursery area for sharks and rays in Northeastern Brazil. Environ Biol Fish 75:349–360
Yokota L, Lessa RP (2007) Reproductive biology of three ray species: Gymnura micrura (Bloch & Schneider, 1801), Dasyatis guttata (Bloch & Schneider, 1801) and Dasyatis marianae Gomes, Rosa & Gadig, 2000, caught by artisanal fisheries in Northeastern Brazil. Cah Biol Mar 48:249–257
Acknowledgements
We thank S. Yoshida (Shimabara Fishermen’s Cooperative, Nagasaki), Y. Kobayashi (Ooura Fishermen’s Cooperative, Saga), Y. Yoshida (Higashiyoka-cho Fisherman’s Cooperative, Saga) and T. Jinkawa (Ashikari Fishermen’s Cooperative, Saga) who supported the collection of specimens. We thank G. Kume (Kagoshima University, Kagoshima), T. Ito, Y. Oka, K. Hara and many students (Nagasaki University, Nagasaki) for their support and research discussions. We appreciate the consideration of two anonymous reviewers for their constructive input. This study was partly supported by a Grant-in-Aid for Scientific Research (B) (No. 23380112) and Grant-in-Aid for Challenging Exploratory Research (No. 26660161) from the Ministry of Education, Culture, Sports, Science and Technology, Japan.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Furumitsu, K., Wyffels, J.T. & Yamaguchi, A. Reproduction and embryonic development of the red stingray Hemitrygon akajei from Ariake Bay, Japan. Ichthyol Res 66, 419–436 (2019). https://doi.org/10.1007/s10228-019-00687-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10228-019-00687-9