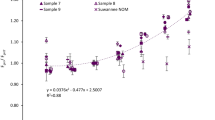
Abstract
Thiols are important antioxidants that can modulate the bioavailability and biogeochemistry of many soft metals, although their detection remains challenging in both their reduced (R–S) and oxidized (R–S–S–R) forms. Here, a modified biochemical method was applied to determine the levels of dissolved and particulate thiols in Lake Biwa water and extracted Lake Biwa fulvic acids obtained at various depths. This method involves the use of the reducing agent tris(2-carboxyethyl)phosphine and the fluorescent label 7-fluorobenzofurazan-4-sulfonic acid ammonium salt (SBD-F), followed by solid-phase extraction and HPLC with fluorescence detection. Dissolved cysteine (Cys) (2.0–6.0 nM), glutathione (GSH) (2.8–5.1 nM), and N-acetyl-l-cysteine (NAC) (1.6–4.2 nM) were detected throughout the water column but were broadly consistent at depths of 5–20 m. In contrast, abundant levels of particulate cysteine (1.3–3.5 × 102 nM) and glutathione (1.6–3.1 × 102 nM) were detected down to depths of 15 m. The particulate cysteine and glutathione were significantly covariant, and the ratios between them reflected the differences in the plankton community composition and availability of these compounds. This work also studied the concentrations of Cys, GSH and NAC in Lake Biwa fulvic acids (LBFAs) for the first time (at 0 m: cysteine, 0.8 nM; glutathione, 1.6 nM; NAC, 2.5 nM; at 10 m: cysteine, 1.4 nM; glutathione, 0.6 nM; NAC, 1.6 nM). The nanomolar to sub-nanomolar concentrations of the particulate and dissolved Cys, GSH and NAC in the lake indicates that these are an important class of ligands for chalcophile metals and may influence the distribution of plankton communities from the epilimnion to the hypolimnion of the lake.




Similar content being viewed by others
References
Aiken GR, McKnight DM, Wershaw RL, MacCarthy P (1985) An introduction to humic substances in soil, sediment, and water. In: Humic substances in soil, sediment, and water: geochemistry, isolation and characterization. Eds. Wiley, New York 1–9
Alwael H, Connolly D, Barron L, Paull B (2010) Development of a rapid and sensitive method for determination of cysteine/cystine ratio in chemically defined media. J Chromatogr A 1217:3863–3870
Amirbahman A, Sigg L, von Gunten U (1997) Reductive dissolution of Fe(III) (hydr)oxides by cysteine: kinetics and mechanism. J Coll Interf Sci 194:194–206
Bertilsson S, Tranvik LJ (2000) Photochemical transformation of dissolved organic matter in lakes. Limnol Oceanogr 45:753–762
Borg H (1994) Trace elements in lakes. In: Salbu B, Steinnes E (eds) Trace elements in natural waters. CRC Press, Boca Raton, pp 177–201
Borisova G, Chukina N, Maleva M, Kumar A, Prasad MNV (2016) Thiols as biomarkers of heavy metal tolerance in the aquatic macrophytes of Middle Urals, Russia. Int J Phytoremed 18:1037–1045
Burns JA, Butler JC, Moran J, Whitesides GM (1991) Selective reduction of disulfides by tris(2-carboxyethyl) phosphine. J Org Chem 56:2648–2650. https://doi.org/10.1021/jo00008a014
Chapman CS, Capodaglio G, Turetta C, van den Berg CMG (2009) Benthic fluxes of copper, complexing ligands and thiol compounds in shallow lagoon waters. Mar Environ Res 67:17–24
Chen W, Li L, Du Z, Liu J, Reitter JN, Mills KV, Linhardt RJ, Wang C (2012) Intramolecular disulfide bond between catalytic cysteines in an intein precursor. J Am Chem Soc 134:2500–2503
Cline DJ, Redding SE, Brohawn SG, Psathas JN, Schneider JP, Thorpe C (2004) New water-soluble phosphines as reductants of peptide and protein disulfide bonds: reactivity and membrane permeability. Biochem 43:15195–15203
Cory RM, McKnight DM, Chin YP, Miller P, Jaros CL (2007) Chemical characteristics of fulvic acids from Arctic surface waters: microbial contributions and photochemical transformations. J Geophys Res 112:G04S51. 10.1029/2006jg000343
Courbot M, Diez L, Ruotolo R, Chalot M, Leroy P (2004) Cadmium-responsive thiols in the ectomycorrhizal fungus Paxillus involutus. Appl Environ Microbiol 70:7413–7417
Cullen WR, Mcbride BC, Reglinski J (1984) The reduction of trimethylarsine oxide to trimethylarsine by thiols—a mechanistic model for the biological reduction of arsenicals. J Inorg Biochem 21:45–60
Dmitrenko O, Thorpe C, Bach RD (2007) The mechanism of SN2 disulfide bond cleavage by phosphorous nucleophiles. Implications for biochemical disulfide reducing agents. J Org Chem 72:8298–8307. https://doi.org/10.1021/jo071271w
Dupont CL, Ahner BA (2005) Effects of copper, cadmium, and zinc on the production and exudation of thiols by Emiliania huxleyi. Limnol Oceanogr 50:508–515
Dupont CL, Nelson R, Bashir S, Moffet J, Ahner B (2004) Novel copper binding and nitrogen-rich thiols produced and exuded by Emiliania huxleyi. Limnol Oceanogr 49:1754–1762
Eitel ME, Taillefert M (2017) Mechanistic investigation of Fe(III) oxide reduction by low molecular weight organic sulfur species. Geochim Cosmochim Acta 215:173–188
Fahey RC, Newton GL (1987) Determination of low-molecular-weight thiols using monobromobimane fluorescent labeling and high-performance liquid chromatography. Methods Enzymol 143:85–96
Fujitake N, Kodama H, Nagao S, Tsuda K, Yonebayashi K (2009) Chemical properties of aquatic fulvic acids isolated from Lake Biwa, a clear water system in Japan. Humic Subs Res 5(6):45–53
Fujitake N, Tsuda K, Aso S, Kodama H, Maruo M, Yonebayashi K (2011) Seasonal characteristics of surface water fulvic acid from Lake Biwa and Lake Tankai in Japan. Limnology 13:45–53
Graneli W, Lindell M, de Faria BM, de Esteves F (1998) Photoproduction of dissolved inorganic carbon in temperate and tropical lakes—dependence on wavelength band and dissolved organic carbon concentration. Biogeochemistry 43:175–195
Haan DH (1993) Solar UV-light penetration and photodegradation of humic substances in peaty lake water. Limnol Oceanogr 38:1072–1076
Haraguchi H, Itoh A, Kimata C, Miwa H (1998) Speciation of yttrium and lanthanides in natural water by inductively coupled plasma mass spectrometry after preconcentration by ultrafiltration and with a chelating resin. Analyst 123:773–778
Hayakawa K (2004) Seasonal variations and dynamics of dissolved carbohydrates in Lake Biwa. Org Geochem 35:169–179
Hori T, Sugiyama Y, Sugiyama M (1998) Chemical and physicochemical characteristics of dissolved organic carbon circulating in harmonic Lake Biwa, Japan. Jpn J Limnol 59:39–52
Hu H, Mylon SE, Benoit G (2006) Distribution of the thiols glutathione and 3-mercaptopropionic acid in Connecticut lakes. Limnol Oceanogr 51:2763–2774
Iglesias A, Lopez R, Fiol S, Antelo JM, Arce F (2003) Analysis of copper and calcium–fulvic acid complexation and competition effects. Water Res 37:3749–3755. https://doi.org/10.1016/s0043-1354(03)00236-7
Irgolic KJ (1991) Determination of organometallic compounds in environmental samples with element-specific detectors. In: Krull IS (ed) Trace metal analysis and speciation. Elsevier, Amsterdam, pp 21–48
Kawakami SK, Achterberg EP (2012) Particulate thiol peptides along a salinity gradient of a metal-contaminated estuary. Estuaries Coasts 35:658–664
Kawakami SK, Gledhill M, Achterberg EP (2006) Determination of phytochelatins and glutathione in phytoplankton from natural waters using HPLC with fluorescence detection. Trends Anal Chem 25:133–142
Kiene RP, Taylor BF (1988) Biotransformations of organosulfur compounds in sediments via 3-mercaptopropionate. Nature 332:148–150
Kiene RP, Malloy KD, Taylor BF (1990) Sulfur containing amino-acids as precursors of thiols in anoxic coastal sediments. App Environ Microbiol 56:156–161
Leal F, Vasconcelos T, van den Berg CMG (2005) Copper-induced release of complexing ligands similar to thiols by Emiliania huxleyi in seawater cultures. Limnol Oceanogr 44:1750–1762
Leclerc M, Planas D, Amyot M (2017) Freshwater sample preservation for the analysis of dissolved low molecular mass thiols. Limnol Oceanogr Methods 15:875–886. https://doi.org/10.1002/lom3.10207
Luther GW, Church TM, Scudlark JR, Cosman M (1986) Inorganic and organic sulfur cycling in salt-marsh pore waters. Science 232:746–749
Mantoura RFC, Dickson A, Kiley JR (1978) The complexation of metals with humic materials in natural waters estuary and coast. Mar Sci 6:387–408
Matrai PA, Vetter RD (1988) Particulate thiols in coastal waters—the effect of light and nutrients on their plankton production. Limnol Oceanogr 33:624–631
Matsunaga K, Igarashi K (1982) Heavy metals and organic complexes (in Japanese). Kaiyo Kagaku. 14:286–291
McKnight DM, Aiken GR (1998) Sources and age of aquatic humus. In: Hessen DO, Tranvik LJ (eds) Aquatic humic substances. Ecological studies (analysis and synthesis), Springer, Berlin, Heidelberg 133: 9–39
McKnight DM, Andrews ED, Aiken GR, Spaulding SA (1994) Aquatic fulvic acids in algal-rich Antarctic ponds. Limnol Oceanogr 39:1972–1979
Meister A, Anderson ME (1983) Glutathione. Ann Rev Biochem 52:711–760
Miseta A, Csutora P (2000) Relationship between the occurrence of cysteine in proteins and the complexity of organisms. Mol Biol Evol 17:1232–1239
Mito S, Sohrin Y, Norisuye K, Matsui M, Hasegawa H, Maruo M, Tsuchiya M, Kawashima M (2004) The budget of dissolved trace metals in Lake Biwa, Japan. Limnology 5:7–16
Mladenov N, Zheng Y, Miller MP, Nemergut DR, Legg T, Simone B, Hageman C, Rahman MM, Ahmed KM, McKnight DM (2010) Dissolved organic matter sources and consequences for iron and arsenic mobilization in Bangladesh aquifers. Environ Sci Technol 44:123–128
Moingt M, Bressac M, Belanger D, Amyot M (2010) Role of ultra-violet radiation, mercury and copper on the stability of dissolved glutathione in natural and artificial freshwater and saltwater. Chemosphere 80:1314–1320
Mopper K, Delmas D (1984) Trace determination of biological thiols by liquid-chromatography and pre-column fluorometric labeling with ortho-phthalaldehyde. Anal Chem 56:2557–2560
Mopper K, Kieber DJ (1991) Distribution and biological turnover of dissolved organic-compounds in the water column of the Black Sea. Deep-Sea Res A 38:S1021–S1047
Perdue EM, Reuter JH, Parrish RS (1984) A statistical model of proton binding by humus. Geochim Cosmochim Acta 48:1257–1263
Rabenstein DL (1989) Metal complexes of glutathione and their biological significance. In: Dolphin D, Avramovic O, Poulson R (eds) Glutathione: chemical, biochemical, and medical aspects. Wiley, New York, pp 147–186
Radwan AF, van den Berg CMG (2001) Thiols in coastal waters of the western North Sea and English Channel. Environ Sci Technol 35:1902–1911
Rigo A, Corazza A, di Paolo ML, Rossetto M, Ugolini R, Scarpa M (2004) Interaction of copper with cysteine: stability of cuprous complexes and catalytic role of cupric ions in anaerobic thiol oxidation. J Inorg Biochem 98:1495–1501
Rigobello ES, Campos SX, Azevedo ERD, Dantas ADB, Vieira EM (2017) Comparative characterization of humic substances extracted from freshwater and peat of different apparent molecular sizes. Rev Ambiente Água 12:774–785. https://doi.org/10.4136/ambi-agua.2022
Robert GMS, Stubbins A, Hernes PJ, Baker A, Mopper K, Aufdenkampe AK, Dyda RY, Mwamba VL, Mangangu AM, Wabakanghanzi JN, Six J (2009) Photochemical degradation of dissolved organic matter and dissolved lignin phenols from the Congo River. J Geophys Res 114:G03010. https://doi.org/10.1029/2009jg000968
Saar RA, Weber JH (1982) Fulvic acid: modifier of metal-ion chemistry. Environ Sci Technol 16:510A–517A. https://doi.org/10.1021/es00103a723
Satoh M, Hirachi Y, Yoshioka A, Kobayashi M, Oyama Y (2002) Determination of cellular levels of nonprotein thiols in phytoplankton and their correlations with susceptibility to mercury. J Phycol 38:983–990
Schaefer JK, Morel FMM (2009) High methylation rates of mercury bound to cysteine by Geobacter sulfurreducens. Nat Geosci 2:123–126
Semeniuk DM, Bundy RM, Payne CD, Barbeau KA, Maldonado MT (2015) Acquisition of organically complexed copper by marine phytoplankton and bacteria in the northeast subarctic Pacific Ocean. Mar Chem 173:222–233
Sohrin Y, Matsui M, Kawashima M, Hojo M, Hasegawa H (1997) Arsenic biogeochemistry affected by eutrophication in Lake Biwa, Japan. Environ Sci Technol 31:2712–2720
Sorensen J (1988) Dimethylsulfide and methane thiol in sediment porewater of a Danish estuary. Biogeochem 6:201–210
Steinberg C (2003) Ecology of humic substances in freshwaters. Springer, Berlin, pp 177–219
Stevenson FJ (1994) Humus chemistry: genesis, composition, reactions, 2nd edn. Wilky, New York
Strasdeit H, Duhme AK, Kneer R, Zenk MH, Hermes C, Nolting HF (1991) Evidence for discrete Cd (SCys)4 units in cadmium phytochelatin complexes from EXAFS spectroscopy. J Chem Soc, Chem Commun 16:1129–1130
Sugiyama Y, Anegawa A, Kumagai T, Harita Y, Hori T, Sugiyama M (2004) Distribution of dissolved organic carbon in various trophic type lakes. Limnology 5:165–176
Sugiyama M, Hori T, Kihara S, Matsui M (2005a) Geochemical behavior of trace elements in Lake Biwa. Limnology 6:117–130
Sugiyama Y, Anegawa A, Inokuchi H, Kumagai T (2005b) Distribution of dissolved organic carbon and dissolved fulvic acid in methotrophic Lake Biwa, Japan. Limnology 6:161–168
Swarr GJ, Kading T, Lamborg CH, Hammerschmidt CR, Bowman KL (2016) Dissolved low-molecular weight thiol concentrations from the U.S. GEOTRACES North Atlantic Ocean zonal transect. Deep-Sea Research I 116:77–87
Tan KH (2003) Humic matter in soil and environment, principles and controversies. Marcel Dekker Inc, New York, p 386
Tang D, Hung CC, Warnken KW, Santschi PH (2000) The distribution of biogenic thiols in surface waters of Galveston Bay. Limnol Oceanogr 45:1289–1297
Tanoue E, Midorikawa T (1995) Detection, characterization and dynamics of dissolved organic ligands in oceanic waters. Biogeochemical processes and ocean flux in the Western Pacific (Sakai H, Nozaki Y eds.), Terra Scientific Publishing, Tokyo 201–224
Thurman EM, Malcolm RL (1981) Preparative isolation of aquatic humic substances. Environ Sci Technol 15:463–466
Tipping E (2002) Cation binding by humic substances. Cambridge University Press, Cambridge
Toyo’oka T, Imai K (1983) High-performance liquid chromatography and fluorometric detection of biologically important thiols, derivatized with ammonium 7-fluorobenzo-2-oxa-1, 3-diazole-4-sulphonate (SBD-F). J Chromatogr 282:495–500
Urabe J, Sekino T, Nozaki K, Tsuji A, Yoshimizu C, Kagami M, Koitabashi T, Miyazaki T, Nakanishi M (1999) Light, nutrients and primary productivity in Lake Biwa: an evaluation of the current ecosystem situation. Ecol Res 14:233–242
Vairavamurthy A, Mopper K (1987) Geochemical formation of organosulfur compounds (thiols) by addition of H2S to sedimentary organic-matter. Nature 329:623–625
Winterbourn CC, Metodiewa D (1999) Reactivity of biologically important thiol compounds with superoxide and hydrogen peroxide. Free Radical Bio Med 27:322–328
Wu F, Tanoue E (2001a) Geochemical characterization of organic ligands for copper(II) in different molecular size fractions in Lake Biwa, Japan. Org Geochem 32:1311–1318
Wu F, Tanoue E (2001b) Molecular mass distribution and fluorescence characteristics of dissolved organic ligands for copper(II) in Lake Biwa, Japan. Org Geochem 32:11–20
Wu F, Midorikawa T, Tanoue E (2001) Fluorescence properties of organic ligands for copper(II) in Lake Biwa and its rivers. Geochem Jour 35:333–346
Xue H, Oestreich A, Kistler D, Sigg L (1996) Free cupric ion concentrations and Cu complexation in selected Swiss lake and rivers. Aquat Sci 58:69–87
Zhang J, Wang F, House DJ, Page B (2004) Thiols in wetland interstitial waters and their role in mercury and methylmercury speciation. Limnol Oceanogr 49:2276–2286
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Yoshiki Sohrin.
Rights and permissions
About this article
Cite this article
Rasheduzzaman, M., Kawaguchi, M., Obata, H. et al. Determination of dissolved and particulate thiols in Lake Biwa water and extracted fulvic acids by solid phase extraction followed by HPLC with fluorescence detection. Limnology 19, 299–309 (2018). https://doi.org/10.1007/s10201-018-0547-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10201-018-0547-1