Abstract
Sexual dimorphism is often derived from sexual selection. In sexually dimorphic Drosophila species, exaggerated male structures are used for specific behaviors in male-to-male competition or courtship toward females. In Drosophila prolongata, a member of the melanogaster species group, males have enlarged forelegs whereas females do not. However, the adaptive role of the enlarged forelegs is unclear because little is known about the behavior of D. prolongata. In this study, the courtship behavior of D. prolongata was investigated in comparison with closely related species. Males of D. prolongata use their forelegs in a specific behavior, “leg vibration”, in which the male vigorously vibrates the female’s abdomen by extending his forelegs from in front of her. Leg vibration was observed immediately before “attempting copulation”, indicating that it has an adaptive role in the mating process. In contrast, leg vibration was not observed in closely related species. Because the large forelegs are necessary to accomplish leg vibration, it was suggested that the sexual dimorphism of D. prolongata forelegs is currently under the influence of sexual selection in courtship behavior.
Similar content being viewed by others
Introduction
Sexual dimorphism, which is frequently expressed in the form of exaggerated structures in males, is often derived from sexual selection (Andersson 1994). It is also presumed that sexually dimorphic structures tend to be accompanied by evolution of specific behavior, in which the exaggerated structures play important roles.
In Drosophila fruit flies, several species have evolved sexually dimorphic structures that are used in specific behaviors. For example, males of Drosophila heteroneura have a broadened head, which is used in male-to-male competition (Spieth 1981; Boake et al. 1997). When males compete for territory, they take up a head-to-head position and push their opponent. The male with a wider head tends to win, and consequently he has a higher probability of mating success (Boake et al. 1997). Another example is seen in male-specific wing pigmentation and courtship behavior among the species that belong to the melanogaster group. In addition to the standard courtship elements in Drosophila, such as orientation, following, wing vibration, and licking (Spieth 1952; Cobb et al. 1986; Yamamoto and Koganezawa 2013), males of species that have wing spots perform “wing display” in front of the female (Fuyama 1979; Yeh et al. 2006), whereas species lacking wing spots do not. Wing spots are associated with wing display in at least seven species that are phylogenetically independent from each other (Yeh et al. 2006). As indicated by these examples, the adaptive roles of sexually dimorphic structures can be better understood in the context of related behavior. In other words, understanding how sexually dimorphic structures are used in the behavioral context gives important insights into the mechanisms by which the morphology evolved through sexual selection.
Drosophila prolongata, a member of the rhopaloa subgroup of the melanogaster species group, is endemic to southwestern China, northeastern India, Myanmar, and Vietnam (Singh and Gupta 1977; Toda 1991; H. Takamori, unpublished observation). The forelegs of D. prolongata are extraordinarily thick and elongated in males. Like other cases of sexual dimorphism, it is presumed that the enlarged forelegs have evolved under sexual selection. However, the adaptive role of the enlarged forelegs is unknown, mostly because of the lack of information on the behavior of D. prolongata. Because such enlarged forelegs have not been observed in any other Drosophila species, it is difficult to infer their function from the known behavior of other species.
In this study, the role of the enlarged forelegs of D. prolongata males in courtship behavior was investigated, with reference to closely related species. We found that the enlarged legs were used in a specific behavior that was observed immediately before attempting copulation, indicating that sexual dimorphism in D. prolongata is under the influence of sexual selection in mating process.
Materials and methods
Fly strains
Drosophila prolongata (BaVi044), D. rhopaloa (BaVi5327), and D. kurseongensis (SaPa058) were collected in Vietnam by H. Takamori in March 2005, September 2004, and March 2009, respectively. Isofemale lines were established by H. Takamori and T. Aotsuka, and maintained at Tokyo Metropolitan University on ordinary cornmeal medium for Drosophila culture. An undescribed species, KB866, was kindly provided by Dr. Artyom Kopp (Barmina and Kopp 2007).
Phylogenetic analysis
The phylogenetic relationships between the four species used in this study were inferred using D. elegans, D. takahashii, D. melanogaster, and D. kikkawai as an outgroup. Four nuclear genes were selected from loci that have been demonstrated to be applicable for phylogenetic analysis of the melanogaster group (Kopp 2006; Yang et al. 2012), comprising extra sexcombs (esc), hunchback (hb), kinase suppressor of ras (ksr), and Phosphoglucose isomerase (Pgi). For each gene, the sequence of the longest exon containing the coding DNA sequence (CDS) was obtained from the Flybase D. melanogaster gene annotation (R5.52). These were FBgn0000588:1, FBgn0001180:2, FBgn0015402:1, and FBgn0003074:4, for esc, hb, ksr, and Pgi, respectively. The corresponding sequences were obtained from the draft genome assembly via the Flybase BLAST web interface for D. takahashii, D. elegans, D. kikkawai, and D. rhopaloa. For the other species, the corresponding sequences were obtained from contigs that were assembled from brain RNA-seq data (accession numbers AB849898–AB849909). Non-protein coding regions and gaps were eliminated. In total, 6,323 sites were included in the analysis. The evolutionary history was inferred using the Maximum Likelihood method based on the Tamura-Nei model with a discrete Gamma distribution model of evolutionary rate differences among sites. The molecular clock was calibrated by the deduced divergence time between D. melanogaster and D. takahashii at 35 million years ago (MYA) (Tamura et al. 2004). All the evolutionary analyses were conducted in MEGA5 (Tamura et al. 2011).
Video recording of courtship behavior
All the species were reared on cornmeal medium at 20 °C in a 12:12 h light:dark cycle. Newly eclosed males and females were maintained separately for 7 days before recording. By this stage, the ovary was fully developed in females of all four species. Courtship behavior was recorded during the period from 1 h after the start of light phase to the end of light phase. A male and a female were introduced into a mating chamber (25 mm in diameter, 15 mm in height) in which a disc of wet filter paper was placed on the bottom. A piece of yeast paste was placed at the center of the chamber. Behavior was recorded using a SONY HDR-CX560V digital camera installed 40 cm above the chamber. Seven chambers were recorded at the same time. Recorded movies were played on PC and inspected visually. The slow-replay function was used occasionally as necessary. Behavioral elements were identified and scored manually.
Transition analysis
For selected pairs, the sequence of behavioral elements was scored for the 15 min preceding successful copulation. At least 30 pairs were scored for each species. Transition matrices are shown in Supplementary Tables S1–S4. Deviation of the frequency of each transition from the expected value was examined by χ 2 test (Hoikkala and Kaneshiro 1993; Chen et al. 2002; Lasbleiz et al. 2006; Jonsson et al. 2011). The expected frequency of transitions was obtained by the method described by Goodman (1968).
For inter-species comparisons, subsequences of behavior that consist of three contiguous behavioral elements were extracted from the video data. In total, 432 patterns of subsequences were identified from the four species. Differences between a target species and the others in the frequency of pairs that exhibited each pattern at least once were examined by Fisher’s exact test with p value adjustment for multiple comparisons by the Bonferroni method.
Results
Morphology and phylogenetic relationship of the species used in this study
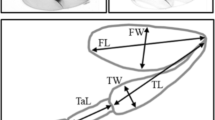
Morphology of the forelegs is sexually dimorphic in D. prolongata (Singh and Gupta 1977; Toda 1991; Figs. 1a, b, 2a, b). Each segment is longer in males, and the femur is thickened along the dorso-ventral axis. The pigmentation pattern is also dimorphic; forelegs have black and white stripes in males. On the other hand, in closely related species, KB866, D. rhopaloa, and D. kurseongensis, the forelegs are monomorphic, as observed in other Drosophila species (Figs. 1c–h, 2c–h).
In contrast to the foreleg morphology, the pigmentation pattern on wings is sexually dimorphic in all four species. D. prolongata has five spots on each wing, which are larger in males (Fig. 3a, b). In KB866, the wings of males are shaded at the front edge, while those of females are occasionally pigmented faintly (Fig. 3c, d). In D. rhopaloa, only males have pale pigmentation on the wings (Fig. 3e, f), and males of D. kurseongensis have a spot at the tip of each wing, whereas this spot is absent in females (Fig. 3g, h).
In spite of these morphological differences, the four species are phylogenetically close to each other. D. kurseongensis was estimated to have diverged from the other three species about 5 MYA, and D. prolongata diverged from KB866 and D. rhopaloa about 4 MYA (Fig. 4), suggesting that the enlarged forelegs in D. prolongata evolved rapidly.
Phylogenetic relationship of the four species used in this study. Drosophila elegans, D. takahashii, D. melanogaster, and D. kikkawai were included as an outgroup. The Maximum Likelihood tree was deduced from the CDS sequences of the four nuclear genes, esc, hb, ksr, and Pgi. The molecular clock was calibrated from the divergence time between D. melanogaster and D. takahashii at 35 MYA (Tamura et al. 2004). Boxes at the internal nodes indicate the standard error
Copulation rate, duration of courtship and copulation
For each of the four species, at least 30 independent courtship episodes ending with successful copulation were recorded. The total number of observed pairs, however, was quite different between species (Table 1). Because of the low copulation rate, a large number of D. prolongata pairs had to be observed to record sufficient number of successful copulations, even though the recording period was set to three times longer (3 h) than that for the other species. Copulation rate was also low in D. kurseongensis (Table 1). Duration of courtship until successful copulation was also different between the species. Most pairs of KB866 and D. rhopaloa copulated within 5 min, but D. prolongata and D. kurseongensis spent much longer in courtship, with larger variations between pairs (significantly different by Bartlett’s test at p = 0.05 level; Table 1; Fig. 5). Although sexual maturation of some Drosophila species, such as D. virilis, is known to require longer period after eclosion (Huttunen et al. 2008), it might not be the reason of low copulation rate in D. prolongata and D. kurseongensis, because the ovary was fully developed by the time of analysis in females of all four species (data not shown).
It is known that the longer copulation delays a female from remating, increasing the likelihood of the male being successful in fathering the offspring under conditions involving sperm competition in D. melanogaster and D. montana (Gilchrist and Partridge 2000; Mazzi et al. 2009). In contrast, the shorter copulation allows females to remate immediately, which benefits females by hedging the risk of mating with a genetically inferior male (Jennions and Petrie 2000). Thus, duration of copulation is thought to be an important parameter resulting from sexual conflict. In many species of the melanogaster group, copulation lasts over 10 min (Hirai et al. 1999; Singh and Singh 2004). Duration of copulation in KB866, D. rhopaloa, and D. kurseongensis was around 15 min, whereas it was about half of that in D. prolongata (Table 1). This result may suggest that the intra- and inter-sexual relationship with regard to sperm competition has changed in D. prolongata. For example, the tendency for remating in females might be different from the other species, although this remains to be confirmed.
Elements of courtship behavior
Behavioral elements were extracted from the recorded courtships. In total, 13 elements were identified (Table 2; Fig. 6; Supplementary Movies S1–S4). Ten elements were commonly observed in the all species. Consistent with their wing pigmentation pattern, they exhibited bi-lateral wing vibration, as reported in other wing-spotted species such as D. suzukii and D. elegans (Fuyama 1979; Yeh et al. 2006).
Leg vibration was observed only in D. prolongata, whereas leg shaking was specific to the other three species (Table 2). Leg vibration is a dynamic movement involving (1) quick positioning in front of the female, (2) extension of both wings, facing their surface towards the female, with wing vibration, and (3) extending both forelegs along the body of the female and vibrating the female’s abdomen violently (Fig. 6c, d; Supplementary Movie S1). This kind of behavior has not been reported in the other Drosophila species. In contrast, leg shaking is different from leg vibration: (1) leg shaking occurs when the male is apart from the female by more than the body length of the fly, (2) in most cases, the male raises and vibrates one foreleg at a time, and (3) the male never touches the female (Supplementary Movies S2–S4).
The frequency of occurrence of each behavioral element is shown in Fig. 7. It should be noted that the elements that appear at earlier stages of courtship may be underrepresented in species that showed longer courtship duration (D. prolongata and D. kurseongensis) because of the limit of the scored period. As in other Drosophila species, uni-lateral wing vibration was the most frequent element in all four species (Fig. 7). Leg display was frequently observed in D. prolongata.
Frequency of occurrence of each behavioral element. Frequency is shown as a proportion of the total number of incidents for each species. The occurrence of each behavioral element was scored for 15 min preceding successful copulation. The number of pairs used in this analysis was the same as that in Fig. 5. The total number of the incidents were; Drosophila prolongata: 2,420; KB866: 841; D. rhopaloa: 418; and D. kurseongensis: 2,497
Transition analysis
Next, transitions between two behavioral elements were analyzed. The standard courtship sequence in Drosophila starts with orientation, followed by tapping, following, uni-lateral wing vibration, and licking, ending with attempting copulation and copulation (Spieth 1952; Cobb et al. 1986; Yamamoto and Koganezawa 2013). Consistent with this, the early stage of courtship in the observed four species seemed to comprise orientation, tapping, and following (Fig. 8). However, the other part of courtship was unique to the observed species, and major differences between D. prolongata and the other three species were seen in this part.
In KB866, D. rhopaloa, and D. kurseongensis, transitions between wing waving, leg shaking, and bi-lateral wing vibration were directionally linked in this order (Fig. 8). Attempting copulation was preceded by uni-lateral wing vibration, consistent with the standard Drosophila courtship. On the other hand, in D. prolongata leg vibration did not form a transition link with wing waving and bi-lateral wing vibration, but it was inserted between uni-lateral wing vibration and attempting copulation. Transition from licking to attempting copulation was also significantly more frequent.
Inter-species comparison of behavioral sequences
Transition diagrams are widely used for the analysis of behavior structures. However, this method has several problems: (1) the analysis is based on single-step transitions between two elements, and transitions that consist of two or more steps cannot be analyzed; (2) transitions between high-frequency elements tend to be underrepresented because the analysis detects the deviation from the proportionally expected frequency of transitions; and (3) the analysis considers the significance among the transitions within a species, and does not support any statistical comparisons between species. To address these problems, we applied a novel method that is able to compare the frequency of two-step-sequences of behavioral elements between species. Briefly, the proportion of the insect pairs that showed a particular two-step-sequence was compared between a species of interest and the others. Behavioral sequences that appeared more or less frequently than in the other species were listed in the order of statistical significance (Table 3). In this analysis, behavioral sequences that contain a species-specific element cannot be compared with other species, because they are always significantly different. For this reason, leg shaking and leg vibration were treated as an equivalent behavioral element, in order to directly compare the behavioral context in which these elements appeared. Thus, the letter “s” represents leg vibration in D. prolongata, whereas it represents leg shaking in the other species.
In D. prolongata, “attempting copulation to copulation (a–c)” was more frequently preceded by leg vibration (s) and less by uni-lateral wing vibration (v) than the other species (Table 3). Conversely, attempting copulation to copulation (a–c) was preceded by uni-lateral wing vibration (v) in KB866 and D. kurseongensis. These results were consistent with the analysis by transition diagrams, and it was confirmed that these elements were actually followed by successful copulations, not by failed attempts. In D. rhopaloa, the frequency of b–a–c (bi-lateral wing vibration to copulation) was significantly higher than in the other species (Table 3).
Because the occurrence of leg display (d) and uni-lateral wing vibration (v) were most frequent in D. prolongata (Fig. 7), transitions between these two elements were underrepresented in the transition diagram (Fig. 8). On the other hand, d–v–d and v–d–v were highly significant in this analysis (Table 3), showing that these transitions are characteristic of D. prolongata courtship. In addition, d–v–e, e–v–d, and v–e–v were significantly more frequent in D. prolongata, showing that elbow rubbing (e) is inserted into uni-lateral wing vibration (v).
In D. kurseongensis, the transition diagram showed that uni-lateral wing vibration (v), wing waving (w), leg shaking (s), and bi-lateral wing vibration (b) were directionally linked in this order (Fig. 8). In Table 3, v–w–s, w–s–b, s–b–v, and b–v–w were significantly more frequent than in the other species, demonstrating that these four elements form a loop of behavioral elements that is characteristic of D. kurseongensis.
The lists of specific sequences for KB866 and D. rhopaloa were shorter than those of the other two species (Table 3). One reason was that their courtship was shorter and consisted of a few courtship elements (on average 25 elements in KB866 and 10 elements in D. rhopaloa, compared with 81 in D. prolongata and 78 in D. kurseongensis), resulting in a smaller repertoire of two-step sequences. At the same time, particularly in D. rhopaloa, behavior was quite variable between individual pairs, making the frequency of each two-step sequence moderate (not extremely high or low) and not significantly different from other species in statistical examination.
Behavioral elements linked to successful copulation
Because the success of copulation is the ultimate objective of the courtship behavior, we counted the all behavioral elements that appeared within the three steps before successful copulation. In KB866 and D. kurseongensis, uni-lateral wing vibration (v) comprised 97.0 and 93.8 %, respectively, of the elements preceding successful copulation (Fig. 9). In D. rhopaloa, uni-lateral wing vibration (v), bi-lateral wing vibration (b), tapping (t), and licking (l) were observed before successful copulation. In D. prolongata, leg vibration (s) was most frequently observed before successful copulation, followed by licking (l), uni-lateral wing vibration (v), and leg display (d) (Fig. 9).
Behavioral elements preceding successful copulation. Behavioral elements that appeared within three steps before successful copulation (c) are shown. Letters in boxes indicate behavioral elements (see Table 2; Fig. 8). The sizes of the boxes represent the proportion of the frequency of each behavioral element. Lines indicate the transition between elements in behavioral sequences. Drosophila prolongata: n = 30; KB866: n = 33; D. rhopaloa: n = 41; D. kurseongensis: n = 32. *In one case in KB866, successful copulation was preceded by only two steps, thus the first element was designated as “−”
Discussion
Functional link between foreleg morphology and leg vibration
In this study, it was revealed that the D. prolongata males use their forelegs in leg vibration during courtship toward females. Such behavior has not been reported in any other Drosophila species. We confirmed that leg vibration was not observed even in the most closely related species, namely, leg vibration is specific to D. prolongata. To accomplish leg vibration, the forelegs need to be long enough to reach the female’s abdomen from in front of her. In this regard, leg vibration appears to be tightly linked with foreleg morphology. In other words, the functional link might underlie the coincidence of long forelegs and leg vibration in D. prolongata.
Effect of leg vibration on copulation success
Although leg vibration was frequently followed by attempting copulation, and thus it seemed to be a kind of signaling behavior from males to females, its effect on copulation success is unknown. Because half of successful copulations were not preceded by leg vibration (Fig. 9), it is clearly dispensable for a sequence of courtship behavior. Among several possibilities, surrounding facts indicate its function in physically stimulating females to increase the rate of copulation success. First, physical stimulation of the female’s abdomen by a male during courtship was reported in several other Drosophila species. In D. silvestris and closely related species, “leg rubbing” behavior was described as an element of courtship (Spieth 1978). In D. virilis, “touching” the female’s abdomen from behind was observed immediately before copulation (Vedenina et al. 2013). These behaviors were thought to stimulate females to accept copulation. Second, a recent study revealed that a vibratory signal was used to immobilize the female during courtship in the species of the melanogaster subgroup (Fabre et al. 2012). Males of these species showed “quivering” of abdomen, by which they produce substrate-borne vibrations that prevent females escaping from courting males. Considering the low copulation rate in D. prolongata (Table 1), males of this species may use leg vibration to make the female more receptive. To understand the function of leg vibration and the effect of leg vibration on female receptivity, as well as the reasons why copulation was not always preceded by leg vibration, remains to be elucidated by further experiments.
Evolution of leg vibration
Evolution of sexual dimorphism is often explained by sexual selection. Because the long forelegs of D. prolongata are used in leg vibration during courtship, it is natural to assume that these characters (morphology and behavior) also evolved under sexual selection. However, it is difficult to infer an evolutionary intermediate state of these characters from the current functional link between them; neither the morphology nor the behavior alone would have been adaptive. For example, leg vibration with short forelegs may not be effective if they do not reach the female’s abdomen. Likewise, long forelegs may not have enough adaptive advantage to balance with their developmental cost without leg vibration. Unfortunately, our observation of closely related species did not provide any insights into this issue, because none of them showed leg vibration. At present, we cannot exclude the possibility that the long forelegs in D. prolongata originally evolved for other reasons, such as a male-to-male aggressive behavior. It is also noteworthy that leg display was a characteristic element in D. prolongata (Fig. 7). Together with the high-contrasting color pattern, the size of the forelegs might have evolved initially as a visual signal. Because leg vibration was dispensable for copulation, it could have evolved after the acquisition of long forelegs. Analysis of the variation among natural populations in foreleg size, as well as in courtship behavior, may provide insights into the evolutionary history of sexual dimorphism and behavior in D. prolongata.
References
Andersson MB (1994) Sexual selection. Princeton University Press, Princeton
Barmina O, Kopp A (2007) Sex-specific expression of a HOX gene associated with rapid morphological evolution. Dev Biol 311:277–286
Boake CRB, DeAngelis MP, Andreadis DK (1997) Is sexual selection and species recognition a continuum? Mating behavior of the stalk-eyed fly Drosophila heteroneura. Proc Natl Acad Sci USA 94:12442–12445
Chen S, Lee AY, Bowens NM, Huber R, Kravitz EA (2002) Fighting fruit flies: a model system for the study of aggression. Proc Natl Acad Sci USA 99:5664–5668
Cobb M, Burnet B, Connolly K (1986) The structure of courtship in the Drosophila melanogaster species sub-group. Behaviour 97:182–211
Fabre CCG, Hedwig B, Conduit G, Lawrence PA, Goodwin SF, Casal J (2012) Substrate-borne vibratory communication during courtship in Drosophila melanogaster. Curr Biol 22:2180–2185
Fuyama Y (1979) A visual stimulus in the courtship of Drosophila suzukii. Experientia 35:1327–1328
Gilchrist AS, Partridge L (2000) Why it is difficult to model sperm displacement in Drosophila melanogaster: the relation between sperm transfer and copulation duration. Evolution 54:534–542
Goodman LA (1968) The analysis of cross-classified data: independence, quasi-independence and interactions in contingency tables with or without missing entries. J Am Stat Assoc 63:1091–1131
Hirai Y, Sasaki H, Kimura MT (1999) Copulation duration and its genetic control in Drosophila elegans. Zool Sci 16:211–214
Hoikkala A, Kaneshiro K (1993) Change in the signal-response sequence responsible for asymmetric isolation between Drosophila planitibia and Drosophila silvestris. Proc Natl Acad Sci USA 90:5813–5817
Huttunen S, Aspi J, Schlötterer C, Routtu J, Hoikkala A (2008) Variation in male courtship song traits in Drosophila virilis: the effects of selection and drift on song divergence at the intraspecific level. Behav Genet 38:82–92
Jennions MD, Petrie M (2000) Why do females mate multiply? A review of the genetic benefits. Biol Rev Camb Philos Soc 75:21–64
Jonsson T, Kravitz EA, Heinrich R (2011) Sound production during agonistic behavior of male Drosophila melanogaster. Fly (Austin) 5:29–38
Kopp A (2006) Basal relationships in the Drosophila melanogaster species group. Mol Phylogenet Evol 39:787–798
Lasbleiz C, Ferveur JF, Everaerts C (2006) Courtship behaviour of Drosophila melanogaster revisited. Anim Behav 72:1001–1012
Mazzi D, Kesäniemi J, Hoikkala A, Klappert K (2009) Sexual conflict over the duration of copulation in Drosophila montana: why is longer better? BMC Evol Biol 9:132
Singh BK, Gupta JP (1977) Two new and two unrecorded species of the genus Drosophila Fallen (Diptera: Drosophilidae) from Shillong, Meghalaya, India. Proc Zool Soc 30:31–38
Singh SR, Singh BN (2004) Female remating in Drosophila: comparison of duration of copulation between first and second matings in six species. Curr Sci 86:465–470
Spieth HT (1952) Mating behavior within the genus Drosophila (Diptera). Bull Am Mus Nat Hist 99:401–474
Spieth HT (1978) Courtship patterns and evolution of the Drosophila adiastola and planitibia species subgroups. Evolution 32:435–451
Spieth HT (1981) Drosophila heteroneura and Drosophila silvestris: head shapes, behavior and evolution. Evolution 35:921–930
Tamura K, Subramanian S, Kumar S (2004) Temporal patterns of fruit fly (Drosophila) evolution revealed by mutation clocks. Mol Biol Evol 21:36–44
Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28:2731–2739
Toda MJ (1991) Drosophilidae (Diptera) in Myanmar (Burma) VII. The Drosophila melanogaster species-group, excepting the D. montium species-subgroup. Orient Insects 25:69–94
Vedenina VY, Ivanova TI, Lazebny OE (2013) Analysis of courtship behavior in closely related species of Drosophila virilis group: a new approach arises new questions. J Insect Behav 26:402–415
Yamamoto D, Koganezawa M (2013) Genes and circuits of courtship behaviour in Drosophila males. Nat Rev Neurosci 14:681–692
Yang Y, Hou ZC, Qian YH, Kang H, Zeng QT (2012) Increasing the data size to accurately reconstruct the phylogenetic relationships between nine subgroups of the Drosophila melanogaster species group (Drosophilidae, Diptera). Mol Phylogenet Evol 62:214–223
Yeh SD, Liou SR, True JR (2006) Genetics of divergence in male wing pigmentation and courtship behavior between Drosophila elegans and D. gunungcola. Heredity 96:383–395
Acknowledgments
This work was supported by JSPS KAKENHI Grant Numbers 23128511 and 25128702 to T.M.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary material 2 (M4 V 9677 kb)
Supplementary material 3 (M4 V 7555 kb)
Supplementary material 4 (M4 V 3804 kb)
Supplementary material 5 (M4 V 12624 kb)
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Setoguchi, S., Takamori, H., Aotsuka, T. et al. Sexual dimorphism and courtship behavior in Drosophila prolongata . J Ethol 32, 91–102 (2014). https://doi.org/10.1007/s10164-014-0399-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10164-014-0399-z