Abstract
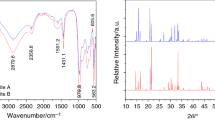
This study suggests a method for the recovery of nitrogen in waste or wastewater as ammonium phosphate crystals. Recovered crystals were a mixture of mono-ammonium phosphate (MAP) and di-ammonium phosphate (DAP), which are valuable fertilizers. A lab-scale system was constructed with an ammonia stripper and absorber with phosphoric acid solution. Formation of ammonium phosphate crystals was dependent on the concentration and volume of phosphoric acid in the absorber. The absorption efficiency of ammonia in the absorber increased as the concentration and volume of phosphoric acid increased. However, the crystals formed better in a smaller volume of absorbing solution. When the N/P ratio in the ammonia absorbed solution reached 0.38 in a continuous stripping condition, crystals could not be formed in the absorber, but could be formed in a crystallizer maintained at 10 °C, the optimal temperature for crystallization. The N/P ratio of the recovered crystals was 1.47, indicating that the crystals were a mixture of MAP and DAP. Analyses of SEM and XRD revealed that the recovered crystals were composed of MAP and DAP.






Similar content being viewed by others
References
Jung JY, Chung YC, Shin HS, Son DH (2004) Enhanced ammonia nitrogen removal using consistent biological regeneration and ammonium exchange of zeolite in modified SBR process. Water Res 38:347–354
Padeyanda Y, Jang YC, Ko Y, Yi S (2016) Evaluation of environmental impacts of food waste management by material flow analysis (MFA) and life cycle assessment (LCA). J mater Cycle Waste Manag 18(3):493–508
Lin L, Chen J, Xu Z, Yuan S, Cao M, Liu H, Lu X (2009) Removal of ammonia nitrogen in wastewater by microwave radiation: a pilot-scale study. J of Hazard Mater 168:862–867
Jiang A, Zhang T, Zhao Q, Frear C, Chen S (2010) Integrated ammonia recovery technology in conjunction with dairy anaerobic digestion. CFF Final Report-AD Component
Ministry of Environment (2014) Environmental statistics yearbook. http://webbook.me.go.kr/DLi-File/091/020/003/5588561.pdf. Accessed 1 Apr 2017
Luo X, Yan Q, Wang C, Luo C, Zhou N, Jian C (2015) Treatment of ammonia nitrogen wastewater in low concentration by two-stage ozonization. Int J Env Res Pub He 12:11975–11987
Yuan MH, Chen YH, Tsai JY, Chang CY (2016) Removal of ammonia from wastewater by air stripping process in laboratory and pilot scales using a rotating packed bed at ambient temperature. J Taiwan Inst Chem E 60:488–495
Bermejo MD, Cantero F, Cocero MJ (2008) Supercritical water oxidation of feeds with high ammonia concentrations: pilot plant experimental results and modeling. Chem Eng J 137:542–549
Choi E, Kim D, EumY Yun Z, Min KS (2005) Full-scale experience for nitrogen removal from piggery waste. Water Environ Res 77:381–389
Uludag-Demirer S, Demirer GN, Chen S (2005) Ammonia removal from anaerobically digested dairy manure by struvite precipitation. Process Biochem 40:3667–3674
Battistoni P, Fava G, Pavan P, Musacco A, Cecchi F (1997) Phosphate removal in anaerobic liquors by struvite crystallization without addition of chemicals: preliminary results. Wat Res 31:2925
Thapa S, Ha TY, Lee H, Adelodun AA (2017) Recovery of ammonium ion as struvite from flue gas scrubbing wastewater. J Mater Cycle Waste Manag. doi:10.1007/s10163-016-0579-8
Gaterell MR, Gay R, Wilson R, Gochin TJ, Lester JN (2000) An economic and environmental evaluation of the opportunities for substituting phosphorus recovered from wastewater treatment works in existing UK fertilizer markets. Environ Technol 21:1067–1084
Gilmour R (2013) Phosphoric acid: purification, uses, technology, and economics, vol 6. CRC Press, Boca Raton, pp 283–284
Gargouri M, Chtara V, Sharrock P, Nzihou A, Feki HA (2012) Experimental study of the purification of an industrial fertilizer (mono-ammonium phosphate) to large scale using an experimental design. Int J Mater Eng 2(4):32–37
Michael R, Cory M (2010) Modeling the cost of production of nitrogen fertilizer produced from wind energy, final report. University of Minnesota, Minnesota
FAO (2015) World fertilizer trends and outlook to 2018, Food and Agriculture Organization, Rome, Italy
Tilman D, Balzer C, Hill J, Befort BL (2011) Global food demand and the sustainable intensification of agriculture. Proc Natl Acad Sci USA 108:20260–20264
Jones A (2016) How phosphate fertilizer prices are changing trends. http://marketrealist.com/2016/03/phosphate-fertilizer-prices-changing-trends/. Accessed 1 Apr 2017
Mubarak YA (2013) Optimum operating conditions for production of crystalline monoammonium phosphate form granulated diammonium phosphate. Arab J Sci Eng 38:777–786
Rabah FKJ, Darwish MS (2013) Characterization of ammonia removal from municipal wastewater using microwave energy: batch experiment. Enviro Nat Resour Res 3(1):42
Walker M, Lyer K, Heaven S, Banks CJ (2011) Ammonia removal in anaerobic digestion by biogas stripping: an evaluation of process alternatives using a first order rate model based on experimental findings. Chem Eng J 178:138–145
Jafari MJ, Ghasemi R, Mehrabi Y, Yazdanbakhsh AR, Hajibabaei M (2012) Influence of liquid and gas flow rates on sulfuric acid mist removal from air by packed bed tower. Iran J Environ Healt 9(1):20
Jones PG (1981) Crystal Growing. Chem Br 17(5):222–225
Gargouri M, Chtara V, Sharrock P, Nzihou A, Feki HA (2014) Purification of an industrial fertilizer using design of experiments. Int J Mater Eng 4:185–191
Cubillas P, Anderson MW (2010) Synthesis mechanism: crystal growth and nucleation. Zeol Catal Synth React Appl 1:1–55
Acknowledgements
This subject was supported by Korea Ministry of Environment (MOE) as “Advanced Technology Program for Environmental Industry” Program (2014000150004) and “Waste to Energy and Recycling Human Resource Development Project”.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dong, D., Choi, O.K., Lee, K. et al. Alternative route for the recovery of nitrogen as ammonium phosphate crystals from high strength waste streams. J Mater Cycles Waste Manag 20, 578–584 (2018). https://doi.org/10.1007/s10163-017-0624-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10163-017-0624-2