Abstract
Background
To improve the long-term outcomes following renal transplantation, prevention of renal-allograft interstitial fibrosis (IF), mainly due to calcineurin inhibitors, is an important therapeutic target. Everolimus (EVR) was reported to have antifibrotic effects. We aimed to investigate the safety, efficacy, and IF of our modified immunosuppressive regimen, which includes early introduction of EVR and reduced-exposure tacrolimus (Tac) (EVR group), and compare it with the standard-exposure tacrolimus-based regimen (Tac group) in de novo living-donor renal recipients.
Methods
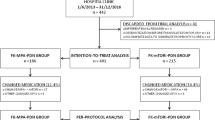
In this retrospective, single-center cohort study, we compared the 2-year clinical courses between the two groups according to intention to treat. Additionally, in patients in whom biopsies were obtained at 1 h, 3 months, and 12 months post-transplant, we compared IF between the groups using imaging analysis.
Results
Overall, 47 patients were included (EVR group, n = 22; Tac group, n = 25). There were no significant differences in renal function and incidences of rejection and viral infections between the groups at the 2-year post-transplant follow-up. However, pathologic imaging analysis (n = 34) revealed chronological progression of IF in the Tac group during the first year post-transplant and no changes in the EVR group (fibrosis rate at 3 months: 20.8 vs. 13.6%, p < 0.001; at 12 months: 24.7 vs. 14.7%, p < 0.001, respectively).
Conclusion
Our modified immunosuppressive regimen may have an antifibrotic effect on transplanted kidneys without loss of safety and efficacy.



Similar content being viewed by others
Change history
26 December 2019
In the Original publication of the article, the co-author name has been misspelled as “Yousuke Nakagawa”.
References
Cosio F, Grande J, Wadei H, Larson T, Griffin M, Stegall M. Predicting subsequent decline in kidney allograft function from early surveillance biopsies. Am J Transplant. 2005;5:2464–72.
Naesens M, Kuypers DR, De Vusser K, et al. Chronic histological damage in early indication biopsies is an independent risk factor for late renal allograft failure. Am J Transplant. 2013;13:86–99.
Shihab FS, Andoh TF, Tanner AM, et al. Role of transforming growth factor-beta 1 in experimental chronic cyclosporine nephropathy. Kidney Int. 1996;49:1141–51.
Alpay N, Ozkok A, Caliskan Y, et al. Influence of conversion from calcineurin inhibitors to everolimus on fibrosis, inflammation, tubular damage and vascular function in renal transplant patients. Clin Exp Nephrol. 2014;18:961–7.
Wang S, Wilkes M, Leof E, Hirschberg R. Noncanonical TGF-beta pathways, mTORC1 and Abl, in renal interstitial fibrogenesis. Am J Physiology Ren Physiology. 2009;298:F142–F149149.
Kurdián M, Herrero-Fresneda I, Lloberas N, et al. Delayed mTOR Inhibition with low dose of everolimus reduces TGFβ expression, attenuates proteinuria and renal damage in the renal mass reduction model. PLoS ONE. 2012;7:e32516.
Rivelli R, Gonçalves R, Leite M, et al. Early withdrawal of calcineurin inhibitor from a sirolimus-based immunosuppression stabilizes fibrosis and the transforming growth factor-β signaling pathway in kidney transplant. Nephrology (Carlton). 2015;20:168–76.
Becker L, Weritz B, Yi X, et al. Evolution of allograft fibrosis and function in kidney transplant recipients: a retrospective analysis of stable patients under CNI and mTORi. Transpl Int. 2015;28:553–64.
Stallone G, Infante B, Schena A, et al. Rapamycin for treatment of chronic allograft nephropathy in renal transplant patients. J Am Soc Nephrol. 2005;16:3755–62.
Witzke O, Sommerer C, Arns W. Everolimus immunosuppression in kidney transplantation: What is the optimal strategy? Transplant Rev. (Orlando). 2016;30:3–12.
Langer R, Hené R, Vitko S, et al. Everolimus plus early tacrolimus minimization: a phase III, randomized, open-label, multicentre trial in renal transplantation. Transpl Int. 2012;25:592–602.
Tedesco Silva H Jr, Cibrik D, Johnston T, et al. Everolimus plus reduced-exposure CsA versus mycophenolic acid plus standard-exposure CsA in renal-transplant recipients. Am J Transplant. 2010;10:1401–13.
Budde K, Becker T, Arns W, et al. Everolimus-based, calcineurin-inhibitor-free regimen in recipients of de-novo kidney transplants: an open-label, randomised, controlled trial. Lancet. 2011;377:837–47.
Holdaas H, Rostaing L, Serón D, et al. Conversion of long-term kidney transplant recipients from calcineurin inhibitor therapy to everolimus: a randomized, multicenter, 24-month study. Transplantation. 2011;92:410–8.
Nara M, Komatsuda A, Numakura K, et al. Quantification of interstitial fibrosis in renal allografts and clinical correlates of long-term graft function. Am J Nephrol. 2017;46:187–94.
Servais A, Meas-Yedid V, Noel LH, et al. Interstitial fibrosis evolution on early sequential screening renal allograft biopsies using quantitative image analysis. Am J Transplant. 2011;11:1456–63.
Matsuo S, Imai E, Horio M, et al. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis. 2009;53:982–92.
Kanda Y. Investigation of the freely-available easy-to-use software “EZR” (Easy R) for medical statistics. Bone Marrow Transplant. 2013;48:452–8.
Vanhove T, Goldschmeding R, Kuypers D. Kidney fibrosis: origins and interventions. Transplantation. 2017;101:713–26.
Schmitt R, Melk A. New insights on molecular mechanisms of renal aging. Am J Transplant. 2012;12:2892–900.
Conway B, Hughes J. Cellular orchestrators of renal fibrosis. QJM. 2012;105:611–5.
Ogawa S, Ishimura T, Miyake H, Fujisawa M. Expression profile of mammalian target of rapamycin-related proteins in graft biopsy specimens: significance for predicting interstitial fibrosis after kidney transplantation. Int J Urol. 2017;24:223–9.
Cosio FG, El Ters M, Cornell LD, Schinstock CA, Stegall MD. Changing kidney allograft histology early posttransplant: prognostic implications of 1-year protocol biopsies. Am J Transplant. 2016;16:194–203.
Acknowledgements
We are grateful to Chisa Okada at the Support Center for Medical Research and Education, Tokai University, for analyzing the images.
Funding
None.
Author information
Authors and Affiliations
Contributions
HI and MN designed the study. HI analyzed the data. HI, MN, SU, ST, MK, NH, YN, TW, MF, and GO performed the study. HI prepared the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have declared that no conflict of interest exists.
Research involving human participants
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee at which the studies were conducted (IRB approval number 18R222) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Ishida, H., Ogura, G., Uehara, S. et al. Preventive effect of early introduction of everolimus and reduced-exposure tacrolimus on renal interstitial fibrosis in de novo living-donor renal transplant recipients. Clin Exp Nephrol 24, 268–276 (2020). https://doi.org/10.1007/s10157-019-01822-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10157-019-01822-6