Abstract
Background
Ceftriaxone (CTRX) is a known cause of biliary pseudolithiasis (BPL) mainly in children. Biliary elimination of CTRX increases in patients with renal dysfunction. However, the influence of renal dysfunction on the incidence of CTRX-associated BPL has not been well investigated. The aim of this study was to investigate the cumulative incidence of CTRX-associated BPL in adults and to assess if renal dysfunction is a risk factor.
Methods
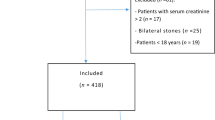
We retrospectively analyzed the medical records of 478 patients treated with CTRX to assess the incidence and risk factors of CTRX-associated BPL. We examined age, sex, body weight, dosage, and duration of CTRX therapy, and the concentrations of serum creatinine, estimated glomerular filtration rate (eGFR), albumin, and serum calcium in all the patients. The cumulative incidence of BPL was calculated using a competing risk model. The multivariate analysis of each variable for the development of BPL was assessed by a Cox proportional hazards model.
Results
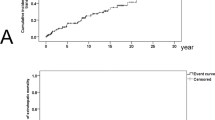
A total of 362 patients (75.7%) had renal dysfunction (eGFR: < 60 mL/min). The cumulative incidence of BPL in patients with renal dysfunction was significantly higher than that in patients with normal kidney function (4.1 vs. 0.6%, p = 0.017). Renal dysfunction (Hazard ratio (HR) 8.14, 95% CI 1.05–63.0, p = 0.045) and female sex (HR 5.35, 95% CI 1.17–24.5, p = 0.031) were independent risk factors of CTRX-associated BPL, which was confirmed using multivariate analysis (renal dysfunction: HR 7.93, 95% CI 1.04–60.5, p = 0.046) (female sex HR 4.65, 95% CI 1.03–21.1, p = 0.046).
Conclusions
Renal dysfunction is an independent risk factor of CTRX-associated BPL in adults.

Similar content being viewed by others
References
Arvidsson A, Alvan G, Angelin B, Borga O, Nord CE. Ceftriaxone: renal and biliary excretion and effect on the colon microflora. J Antimicrob Chemother. 1982;10:207–15.
Richards DM, Heel RC, Brogden RN, Speight TM, Avery GS. Ceftriaxone: a review of its antibacterial activity, pharmacological properties and therapeutic use. Drugs. 1984;27:469–527.
Park HZ, Lee SP, Schy AL. Ceftriaxone-associated gallbladder sludge. Identification of calcium-ceftriaxone salt as a major component of gallbladder precipitate. Gastroenterology. 1991;100:1665–70.
Shiffman ML, Keith FB, Moore EW. Pathogenesis of ceftriaxone-associated biliary sludge. In vitro studies of calcium-ceftriaxone binding and solubility. Gastroenterology. 1990;99:1772–8.
Schaad UB, Tschappeler H, Lentze MJ. Transient formation of precipitations in the gallbladder associated with ceftriaxone therapy. Pediatr Infect Dis. 1986;5:708–10.
Schaad UB, Wedgwood-Krucko J, Tschaeppeler H. Reversible ceftriaxone-associated biliary pseudolithiasis in children. Lancet. 1988;2:1411–3.
Schaad UB, Suter S, Gianella-Borradori A, Pfenninger J, Auckenthaler R, Bernath O, et al. A comparison of ceftriaxone and cefuroxime for the treatment of bacterial meningitis in children. N Engl J Med. 1990;322:141–7.
Papadopoulou F, Efremidis S, Karyda S, Badouraki M, Karatza E, Panteliadis C, et al. Incidence of ceftriaxone-associated gallbladder pseudolithiasis. Acta Paediatr. 1999;88:1352–5.
Palanduz A, Yalcin I, Tonguc E, Guler N, Ones U, Salman N, et al. Sonographic assessment of ceftriaxone-associated biliary pseudolithiasis in children. J Clin Ultrasound. 2000;28:166–8.
Ozturk A, Kaya M, Zeyrek D, Ozturk E, Kat N, Ziylan SZ. Ultrasonographic findings in ceftriaxone: associated biliary sludge and pseudolithiasis in children. Acta Radiol. 2005;46:112–6.
Biner B, Oner N, Celtik C, Bostancioglu M, Tuncbilek N, Guzel A, et al. Ceftriaxone-associated biliary pseudolithiasis in children. J Clin Ultrasound. 2006;34:217–22.
Kong MS, Chen CY. Risk factors leading to ceftriaxone-associated biliary pseudolithiasis in children. Changgeng Yi Xue Za Zhi. 1996;19:50–4.
Soysal A, Erasov K, Akpinar I, Bakir M. Biliary precipitation during ceftriaxone therapy: frequency and risk factors. Turk J Pediatr. 2007;49:404–7.
Pigrau C, Pahissa A, Gropper S, Sureda D, Martinez Vazquez JM. Ceftriaxone-associated biliary pseudolithiasis in adults. Lancet. 1989;2:165.
Heim-Duthoy KL, Caperton EM, Pollock R, Matzke GR, Enthoven D, Peterson PK. Apparent biliary pseudolithiasis during ceftriaxone therapy. Antimicrob Agents Chemother. 1990;34:1146–9.
Cometta A, Gallot-Lavallee-Villars S, Iten A, Cantoni L, Anderegg A, Gonvers JJ, et al. Incidence of gallbladder lithiasis after ceftriaxone treatment. J Antimicrob Chemother. 1990;25:689–95.
Becker CD, Fischer RA. Acute cholecystitis caused by ceftriaxone stones in an adult. Case Rep Med. 2009;2009:132452.
Zinberg J, Chernaik R, Coman E, Rosenblatt R, Brandt LJ. Reversible symptomatic biliary obstruction associated with ceftriaxone pseudolithiasis. Am J Gastroenterol. 1991;86:1251–4.
Bickford CL, Spencer AP. Biliary sludge and hyperbilirubinemia associated with ceftriaxone in an adult: case report and review of the literature. Pharmacotherapy. 2005;25:1389–95.
Stoeckel K, McNamara PJ, Hoppe-Seyler G, Blumberg A, Keller E. Single-dose ceftriaxone kinetics in functionally anephric patients. Clin Pharmacol Ther. 1983;33:633–41.
Bor O, Dinleyici EC, Kebapci M, Aydogdu SD. Ceftriaxone-associated biliary sludge and pseudocholelithiasis during childhood: a prospective study. Pediatr Int. 2004;46:322–4.
Sasaki Y, Aoki S, Aoki K, Achiwa K, Yama T, Kubota M, et al. Acute pancreatitis associated with the administration of ceftriaxone in an adult patient. Nihon Shokakibyo Gakkai Zasshi. 2009;106:569–75.
Stoeckel K, Koup JR. Pharmacokinetics of ceftriaxone in patients with renal and liver insufficiency and correlations with a physiologic nonlinear protein binding model. Am J Med. 1984;77:26–32.
Cohen D, Appel GB, Scully B, Neu HC. Pharmacokinetics of ceftriaxone in patients with renal failure and in those undergoing hemodialysis. Antimicrob Agents Chemother. 1983;24:529–32.
Rivera M, Garcia-Herrera AL, Burguera V, Sosa-Barrios H, Palomares JR, Quereda C. Ceftriaxone-associated gallbladder pseudolithiasis in a PD patient. Perit Dial Int. 2011;31:97–9.
Kutuya N, Ozaki Y, Okazaki T. A symptomatic child with ceftriaxone-associated biliary pseudolithiasis. J Med Ultrason. 2008;35:125–8.
Robertson FM, Crombleholme TM, Barlow SE, Verhave M, Brown D. Ceftriaxone choledocholithiasis. Pediatrics. 1996;98:133–5.
Acknowledgements
This study was funded by the Okinaka Memorial Institute for Medical Research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have declared that no conflict of interest exists.
Human and animal rights
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee at which the studies were conducted (IRB Approval Number 994) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
About this article
Cite this article
Imafuku, A., Sawa, N., Sekine, A. et al. Risk factors of ceftriaxone-associated biliary pseudolithiasis in adults: influence of renal dysfunction. Clin Exp Nephrol 22, 613–619 (2018). https://doi.org/10.1007/s10157-017-1493-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10157-017-1493-7