Abstract
Background
Currently kidney disease appears a foremost problem across the world. Acetaminophen is a commonly used antipyretic agent, which in high doses, causes uremia and used for experimentally induction of kidney disease. Bacteriotherapy affords a promising approach to mitigate uremic toxins by ingestion of urease positive bacteria, probiotics and symbiotic able to catabolize uremic solutes within the gut. The present study evaluates the effect of seven commercial symbiotic on kidney disease.
Methods
Fifty-four albino male rats were randomly divided into nine groups. Control group (Group-I) received distilled water interperitoneally for 7 days. Positive control group (Group-II) received 500 mg/kg acetaminophen interperitoneally for 7 days. Commercially available seven symbiotic combinations at a dose of 109cells/day for 3 weeks was administered to the tested groups (Group III–IX) after receiving 500 mg/kg/day acetaminophen interperitoneally for 7 days. Blood, kidney, liver and stool samples were collected after scarification for biochemical tests and DNA fragmentation assay of kidney tissue, kidney histological studies. Limited fecal analysis was conducted.
Result
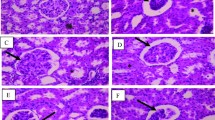
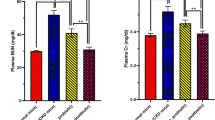
Blood urea nitrogen and toxicity indicators were increased, and antioxidant enzymes were decreased in Group-II. Blood urea nitrogen, toxicity indicators, glomerular necrosis, DNA damage of kidney tissue were reduced, and antioxidant enzymes were increased significantly in the treated Groups IV and IX (p < 0.05) in response to Group-II. Number of pathogenic bacteria decreased in synbiotic treated groups than Group I and II.
Conclusion
The study demonstrated that some of commercial symbiotic combination can reduce the sever effect of kidney disease.


Similar content being viewed by others
References
Ayodele OE, Alebiosu CO. Burden of chronic kidney disease: an international perspective. Adv Chronic Kidney Dis. 2010;17:215–24.
Agarwal SK, Dash SC, Irshad M, et al. Prevalence of chronic renal failure in adults in Delhi, India. Nephrol Dial Transplant. 2005;20:1638–42.
Nelson SD. Mechanisms of the formation and disposition of reactive metabolites that can cause acute liver injury. Drug Metab Rev. 1995;27:147–77.
Jones AF, Vale JA. Paracetamol poisoning and the kidney. J Clin Pharm Ther. 1993;18:5–8.
Corina L, Pilar J, Ana S, Dolores S, Jesu´ SE, Alberto O. Paracetamol-induced renal tubular injury: a role for ER stress. J Am Soc Nephrol. 2004;15:380–9.
Hart SG, Beierschmitt WP, Wyand DS, et al. Acetaminophen nephrotoxicity in CD-1 mice.I. Evidence of a role for in situ activation in selective covalent binding and toxicity. Toxicol Appl Pharmacol. 1994;126:267–75.
Sparks RE. Review of gastrointestinal perfusion in the treatment of uremia. Clin Nephrol. 1979;11:81–5.
Hida M, Aiba Y, Sawamura S. Inhibition of the accumulation of uremic toxins in the blood and their precursors in the feces after oral administration of Lebenin®, a lactic acid bacteria preparation, to uremic patients undergoing hemodialysis. Nephron. 1996;74:349–55.
Patel BG, Zelenaia OZ, Dhree RS, Natarajan R, Friedman EA. Oral bacteriotherapy effectively reduces severity of azotemia in 5/6th nephrectomized rats. J Am Soc Nephrol. 2003;14:765.
Dunn SR, Marczely J, Ranganathan P, Michael LS, Friedman EA. Probiotics extend survival in untreated 5/6th nephrectomized rats: possible use for probiotics as an adjunct in chronic renal failure (CRF). J Am Soc Nephrol. 2003;14:765.
Ranganathan N, Patel B, Ranganathan P. Probiotic amerlioration of azotemia in 5/6th nephrectomized Sprague-Dawley rats. Sci World J. 2005;5:652–60.
Mandal A, Das K, Roy S, Mondal KCh, Nandi DK. In vivo assessment of bacteriotherapy on acetaminophen-induced uremic rats. J Nephrol. 2013;26(1):228–36.
Mandal A, Mandal S, Roy S, et al. Assessment of efficacy of a potential probiotic strain and its antiuremic and antioxidative activities. e-SPEN Journal. (in press) doi:10.1016/j.clnme.2013.05.001.
Olert ED, Cross BM, McWilliam AA. Guide to Care and Use of Experimental Animals. In: Olert ED, McWilliam BM, eds. Canadian Council on Animal Care. 2nd ed. Ottawa. 19931-90.
Burtis CA, Ashwood ER, Tietz, 3rd ed. Textbook of Clinial Chemistry. Philadelphia, WB Saunders Company; (1999).
Sabbagh M, Rick W, Schneide RS. A kinetic method for the direct determination of creatinine in serum with 3,5-dinitrobenzoic acid without deproteinization. J Clin Chem Clin Biochem. 1988;26:15–24.
Beers RF, Sizer IW. A spectrophotometric method for measuring the breakdown of hydrogen peroxide of catalase. J Biol Chem. 1952;195:133–40.
Das J, Ghosh J, Manna P, Sinha M, Sil PC. Arsenic-induced oxidative cerebral disorders: protection by taurine. Drug Chem Toxicol. 2009;32:93–102.
Marklund S, Marklund G. Involvement of superoxide anion radical in autotoxidation of pyrogallol and a convenient assay of superoxide dismutase. Eur J Biochem. 1974;47:469–74.
Ellman GL. Tissue sulphydryl groups. Arch Biochem. 1959;82(1):70–7.
Tawfik DS. Modification of sulfhydryl groups with DTNB. In: Walker JM, editor. The protein protocols handbook. Hatfield, UK: Humana Press; 1996. p. 367–8.
Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxidation in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–8.
Goel BK. Routine biochemical test. In: Mukherjee KL, editor. Medical laboratory technology. New Delhi: Tata McGraw-Hill Publishing Company Ltd; 1988. p. 985–1079.
Lin KT, Xue JY, Sun FF, Wong PYK. Reactive oxygen species participate in peroxinitrile induced apoptosis in HL 60 cells. Biochem Biophys Res Commun. 1997;230:115–9.
Das J, Ghosh J, Manna P, Sil PC. Taurine protects acetaminophen-induced oxidative damage in mice kidney through APAP urinary excretion and CYP2E1 inactivation. Toxicology. 2010;269:24–34.
Bennit WM, Parker RA, Elliot WC, et al. Sex related differences in the susceptibility of rat to gentamicin nephrotoxicity. J Infect Dis. 1982;145:370–4.
Guyton AC. Red Blood Cells, Anemia, and Polycythemia, eds. In: Textbook of Medical Physiology. 8th ed. Philadelphia, WB Saunders Co., (1991) p 56–64.
Mayne PD. The kidneys and renal calculi, eds. In: Clinical chemistry in diagnosis and treatment. 6th ed. London, Edward Arnold Publications, (1994) p 2–24.
Ali BH, Ben Ismail TH, Basheer AA. Sex related differences in the susceptibility of rat to gentamicin nephrotoxicity: influence of gonadectomy and hormonal replacement therapy. Ind J of Pharmacol. 2001;33:369–73.
Somani SM, Husain K, Whitworth C, et al. Dose-dependent protection by lipoicacid against cisplatin-induced nephrotoxicity in rats: antioxidant defense system. Pharmacol Toxicol. 2000;86:234–41.
Abdel-Zaher OA, Abdel-Rahman MM, Hafez MM. Role of nitric oxide and reduced glutathione in the protective effects of aminoguanidine, gadolinium chloride and oleanolic acid against acetaminophen-induced hepatic and renal damage. Toxicology. 2007;243:124–34.
Lin MY, Yen CL. Inhibition of lipid peroxidation by Lactobtacillus acidophilus and bifidobacterium longum. J Agric Food Chem. 1999;47:3661–4.
Mandal A, Paul T, Roy S. Effect of newly isolated Lactobacillus ingluviei ADK10, from chicken intestinal tract on acetaminophen induced oxidative stress in Wistar rats. Indian J Exp Biol. 2013;51(02):174–80.
Pande M, Flora SJ. Lead induced oxidative damage and its response to combined administration of alpha-lipoic acid and succimers in rats. Toxicology. 2002;17:187–96.
Manov I, Hirsh M, Ianccu TC. Acetaminophen hepatotoxicity and mechanisms of its protection by N-acetylcysteine: a studyof Hep3B cells. Exp Toxicol Pathol. 2003;53:489–500.
Pradhan S, Mandal S, Roy S, Mandal A, Das K, Nandi DK. Attenuation of uremia by orally feeding alpha-lipoic acid on acetaminophen induced uremic rats. Saudi Pharm J. 2013;21:187–92.
Ouwehand AC, Vesterlund S. 11 Antimicrobial components from lactic acid bacteria. Salminen S, Ouwehand A, Von Wright A, eds. Lactic Acid Bacteria: Microbial and Functional Aspects. 3rd ed. New York, Marcel Dekker. 375–395 (2004).
Gomes DA, Souza AML, Lopes RV, Nunes AC, Nicoli RJ. Comparison of antagonistic ability against enteropathogens by G+ and G− anaerobic dominant components of human fecal microbiota. Folia Microbiol. 2006;51:141–5.
Acknowledgments
The authors grateful to authority of Department of science and technology (DST), government of India for providing fund as INSPIRE Fellowship for this work.
Conflict of interest
All the authors have declared no competing interest.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Mandal, A., Patra, A., Mandal, S. et al. Therapeutic potential of different commercially available synbiotic on acetaminophen-induced uremic rats. Clin Exp Nephrol 19, 168–177 (2015). https://doi.org/10.1007/s10157-014-0971-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10157-014-0971-4