Abstract
Background
Prorenin, the precursor of renin, binds to the (pro)renin receptor [(P)RR] and triggers intracellular signaling. The ligand binding sites of (P)RR are disconnected and are present in the soluble form of the receptor in serum. Given that the clinical significance of serum prorenin and soluble (P)RR in chronic kidney disease (CKD) is unclear, we investigated the relationship between serum prorenin, soluble (P)RR, and various clinical parameters in patients with CKD.
Methods
A total of 374 patients with CKD were enrolled. Serum samples were collected, and the levels of soluble (P)RR and prorenin were measured using ELISA kits. Serum creatinine (Cr), blood urea nitrogen (BUN), uric acid (UA), hemoglobin (Hb), soluble secreted α-Klotho, and the urine protein/Cr ratio were also measured. Similarly, clinical parameters were also evaluated using serum and urine sample collected after 1 year (n = 204).
Results
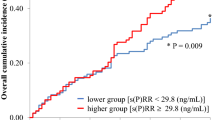
Soluble (P)RR levels were positively associated with serum Cr (P < 0.0001, r = 0.263), BUN (P < 0.0001, r = 0.267), UA (P < 0.005, r = 0.168) levels, CKD stage (P < 0.0001, r = 0.311) and urine protein/Cr ratio (P < 0.01, r = 0.157), and inversely with estimated glomerular infiltration rate (eGFR) (P < 0.0001, r = −0.275) and Hb (P < 0.005, r = −0.156). Soluble (P)RR levels were inversely associated with α-Klotho levels (P < 0.001, r = −0.174) but did not correlate with prorenin levels. With respect to antihypertensive drugs, soluble (P)RR levels were significantly lower in patients treated with an angiotensin II receptor blocker (ARB) than in those without ARB therapy (P < 0.005). Soluble (P)RR levels were significantly lower in CKD patients with diabetes mellitus or primary hypertension than in those without these conditions (P < 0.05). In contrast, serum levels of prorenin did not correlate with parameters related to renal function. Serum prorenin levels were significantly higher in CKD patients with diabetes mellitus than in nondiabetic patients (P < 0.05), but not in CKD patients with hypertension (P = 0.09). Finally, with respect to the relationship between basal soluble (P)RR levels and the progression rates of renal function, soluble (P)RR levels were positively associated with ΔCr (P < 0.05, r = 0.159) and inversely associated with ΔeGFR (P < 0.05, r = −0.148).
Conclusion
Serum levels of soluble (P)RR correlated with the stage of CKD. Our findings suggest that soluble (P)RR may be involved in renal injury and influence the progression of CKD.








Similar content being viewed by others
References
Weir MR, Dzau VJ. The renin–angiotensin–aldosterone system: a specific target for hypertension management. Am J Hypertens. 1999;12:205S–13S.
Kim S, Iwao H. Molecular and cellular mechanisms of angiotensin II-mediated cardiovascular and renal diseases. Pharmacol Rev. 2000;52:11–34.
Dzau VJ, Bernstein K, Celermajer D, Cohen J, Dahlöf B, Deanfield J, Diez J, Drexler H, Ferrari R, van Gilst W, Hansson L, Hornig B, Husain A, Johnston C, Lazar H, Lonn E, Lüscher T, Mancini J, Mimran A, Pepine C, Rabelink T, Remme W, Ruilope L, Ruzicka M, Schunkert H, Swedberg K, Unger T, Vaughan D, Weber M. The relevance of tissue angiotensin-converting enzyme: manifestations in mechanistic and endpoint data. Am J Cardiol. 2001;88:1L–20L.
Derkx FH, Schalekamp MA. Human prorenin: pathophysiology and clinical implications. Clin Exp Hypertens A. 1988;10:1213–25.
Luetscher JA, Kraemer FB, Wilson DM, Schwartz HC, Bryer-Ash M. Increased plasma inactive renin in diabetes mellitus. A marker of microvascular complications. N Engl J Med. 1985;312:1412–7.
Wilson DM, Luetscher JA. Plasma prorenin activity and complications in children with insulin-dependent diabetes mellitus. N Engl J Med. 1990;323:1101–6.
Nguyen G, Delarue F, Berrou J, Rondeau E, Sraer JD. Specific receptor bindingofreninonhuman mesangial cells in cultureincreasesplasminogen activator-1 antigen. Kidney Int. 1996;50:1897–903.
Nguyen G, Delarue F, Burcklé C, Bouzhir L, Giller T. SraerJD. Pivotal role of the renin/prorenin receptor in angiotensin II production and cellular responses to renin. J Clin Invest. 2002;109:1417–27.
Huang Y, Wongamorntham S, Kasting J, McQuillan D, Owens RT, Yu L, Noble NA, Border W. Renin increases mesangial cell transforming growth factor-beta1 and matrix proteins through receptor-mediated, angiotensin II-independent mechanisms. Kidney Int. 2006;69:105–13.
Matsuo S, Imai E, Horio M, Yasuda Y, Tomita K, Nitta K, Yamagata K, Tomino Y, Yokoyama H, Hishida A. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis. 2009;53:982–92.
Schalekamp MADH, Derkx FH, Deinum J, Danser AJ. Newly developed rennin and prorenin assays and the clinical evaluation of rennin inhibitors. J Hypertens. 2008;26:928–37.
Watanabe N, Bokuda K, Fujiwara T, Suzuki T, Mito A, Morimoto S, Jwa SC, Egawa M, Arai Y, Suzuki F, Sago H, Ichihara A. Soluble (pro)renin receptor and blood pressure during pregnancy: a prospective cohort study. Hypertension. 2012;60:1250–6.
Advani A, Kelly DJ, Cox AJ, White KE, Advani SL, Thai K, Connelly KA, Yuen D, Trogadis J, Herzenberg AM, Kuliszewski MA, Leong-Poi H, Gilbert RE. The (Pro)renin receptor: site-specific and functional linkage to the vacuolar H+–ATPase in the kidney. Hypertension. 2009;54:261–9.
Hirose T, Mori N, Totsune K, Morimoto R, Maejima T, Kawamura T, Metoki H, Asayama K, Kikuya M, Ohkubo T, Kohzuki M, Takahashi K, Imai Y. Increased expression of (pro)renin receptor in the remnant kidneys of 5/6 nephrectomized rats. Regul Pept. 2010;159:93–9.
Ichihara A, Itoh H, Inagami T. Critical roles of (pro)renin receptor-boundprorenin in diabetes andhypertension: salliesintotherapeutic approach. J Am Soc Hypertens. 2008;2:15–9.
Cruciat CM, Ohkawara B, Acebron SP, Karaulanov E, Reinhard C, Ingelfinger D, Boutros M, Niehrs C. Requirement of prorenin receptor and vacuolar H+-ATPase-mediated acidification for Wnt signaling. Science. 2010;327:459–63.
Gonzalez AA, Lara LS, Luffman C, Seth DM, Prieto MC. Soluble form of the (pro)renin receptor is augmented in the collecting duct and urine of chronic angiotensin II-dependent hypertensive rats. Hypertension. 2011;57:859–64.
Ichihara A. (Pro)renin receptor and autophagy in podocytes. Autophagy. 2012;8:271–2.
Schefe JH, Menk M, Reinemund J, Effertz K, Hobbs RM, Pandolfi PP, Ruiz P, Unger T, Funke-Kaiser H. A novel signal transduction cascade involving direct physical interaction of the renin/prorenin receptor with the transcription factor promyelocytic zinc finger protein. Circ Res. 2006;99:1355–66.
Nguyen G, Contrepas A. Physiology and pharmacology of the (pro)renin receptor. Curr Opin Pharmacol. 2008;8:127–32.
Siragy HM, Huang J. Renal (pro)renin receptor regulation in diabetic ratsthrough enhanced angiotensin AT1 receptor and NADPH oxidase activity. Exp Physiol. 2008;93:709–14.
Kurtz A, Wagner C. Regulation of renin secretin by angiotensin II-AT1 receptor. J Am Soc Nephrol. 1999;19:162–8.
Siragy HM, Awad A, Abadir P, Webb R. The angiotensin II type 1 receptor mediates renal interstitial content of tumor necrosis factor-α in diabetic rats. Endocrinology. 2003;144:2229–33.
Sodhi CP, Kanwar YS, Sahai A. Hypoxia and high glucose upregulate AT1 receptor expression and potentiate ANG II-induced proliferation in VSM cells. Am J Physiol Heart Circ Physiol. 2003;284:846–52.
Privratsky JR, Wold LE, Sowers JR, Quinn MT, Ren J. AT1 blockade prevents glucose-induced cardiac dysfunction in ventricular myocytes: role of the AT1 receptor and NADPH oxidase. Hypertension. 2003;42:206–12.
Onozato ML, Toji A, Goto A, Fujita T, Wilcox CS. Oxidative stress and nitric oxide synthase in rat diabetic nephropathy: effects of ACEI and ARB. Kidney Int. 2002;61:186–94.
Ferri N, Greco CM, Corsini GMA. Aliskiren reduces prorenin receptor expression and activity in cultured human aortic smooth muscle cells. J Renin Angiotensin Aldosterone Syst. 2011;12:469–74.
Feldman DL, Jin L, Xuan H, Contrepas A, Zhou Y, Webb RL, Mueller DN, Feldt S, Cumin F, Maniara W, Persohn E, Schuetz H, Jan Danser AH, Nguyen G. Effects of aliskiren on blood pressure, albuminuria, and (pro)renin receptor expression in diabetic TG(mRen-2)27 rats. Hypertension. 2008;52:130–6.
Lu H, Rateri DL, Feldman DL, Jr RJ, Fukamizu A, Ishida J, Oesterling EG, Cassis LA, Daugherty A. Renin inhibition reduces hypercholesterolemia-induced atherosclerosis in mice. J Clin Invest. 2008;118:984–93.
Brown MJ. Aliskiren. Circulation. 2008;118:773–84.
Acknowledgments
This work was supported by the Japan Osteoporosis Foundation, Japanese Kidney Foundation, Kochi Organization for Medical Reformation and Renewal (to YT), and a grant from the Ministry of Education, Science, Culture and Sports of the Japan government (to YS, KI, KO, SF, and YT). We thank Ms. Reiko Matsumoto, Ms. Akiko Takano, and Ms. Sekie Saito for their technical assistance.
Conflict of interest
All the authors have declared no competing interest.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Hamada, K., Taniguchi, Y., Shimamura, Y. et al. Serum level of soluble (pro)renin receptor is modulated in chronic kidney disease. Clin Exp Nephrol 17, 848–856 (2013). https://doi.org/10.1007/s10157-013-0803-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10157-013-0803-y