Abstract
Background
Pulmonary-renal syndrome is characterized by pulmonary hemorrhage and rapidly progressive glomerulonephritis in various immunological states. Histopathological analysis of pulmonary-renal syndrome is not yet complete.
Methods
Wistar–Kyoto (WKY) rats were sensitized using the noncollagenous (NC1) domain of type IV collagen from bovine kidney as an antigen. Histopathology of the kidneys and lungs was investigated with light microscopy, immunohistochemistry and electromicroscopy. Expression levels of cytokine mRNA were determined by real-time RT-PCR using renal tissue of rats.
Results
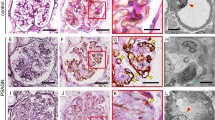
Macrophage-rich granulomatous glomerulonephritis and alveolar capillaritis accompanied with pulmonary hemorrhage were induced by the sensitization. The humoral antibody against NC1 was detected on the glomerular and alveolar capillary walls. Th2 cytokine IL-10 was dominant over Th1 cytokine IFN-γ in renal tissues of WKY rats.
Conclusion
The granulomatous transformation seemed to be induced by macrophage conspicuous capillaritis under dominant cellular immune reactions in WKY rats. In addition to Th1 cytokines, Th2 cytokines may also participate in the formation of granulomatous lesions.









Similar content being viewed by others
References
Glassock RJ, Cohen AH, Adler SG, Ward HJ. Goodpasture’s disease. In: Brenner BM, Rector FC, editors. The kidney. 4th ed. Philadelphia: WB Saunders; 1991. p. 1301–5.
Shigematsu H, Kobayashi Y. Pulmonary involvements in the initial phase of rat Masugi nephritis. Virchows Arch B Cell Pathol. 1972;11:111–23.
Shigematsu H. Glomerular events during the initial phase of rat Masugi nephritis. Virchows Arch B Cell Pathol. 1970;5:187–200.
Kawasaki K, Yaoita E, Yamamoto T, Kihara I. Depletion of CD8 positive cells in nephrotoxic serum nephritis of WKY rats. Kidney Int. 1992;41:1517–26.
Abbate M, Kalluri R, Corna D, Yamaguchi N, McCluskey RT, Hudson BG, et al. Experimental Goodpasture’s syndrome in Wistar–Kyoto rats immunized with α3 chain of type IV collagen. Kidney Int. 1998;54:1550–61.
Hoffmann KF, Caspar P, Cheever AW, Wynn TA. IFN-γ, IL-12, and TNF-α are required to maintain reduced liver pathology in mice vaccinated with Schistosoma mansoni eggs and IL-12. J Immunol. 1998;161:4201–10.
Müller A, Trabandt A, Gloeckner-Hofmann K, Seitzer U, Csernok E, Schönermarck U, et al. Localized Wegener’s granulomatosis: predominance of CD26 and IFN-γ expression. J Pathol. 2000;192:113–20.
Shigematsu H, Kobayashi Y. The development and fate of the immune deposits in the glomerulus during the secondary phase of rat Masugi nephritis. Virchows Arch B Cell Pathol. 1971;8:83–95.
Mukai K, Shibata T, Kato K, Sugisaki T. Adjuvant-induced macrophage-dominant nephrotoxic serum nephritis in rats. Clin Exp Nephrol. 2005;9:15–23.
Leatherman JW, Sibley RK, Davies SF. Diffuse intrapulmonary hemorrhage and glomerulonephritis unrelated to anti-glomerular basement membrane antibody. Am J Med. 1982;72:401–10.
Churg J, Bernstein J, Glassock RJ. Glomerular lesions in vascular diseases. In: Churg J, Bernstein J, Glassock RJ, editors. Renal diseases: classification and atlas of glomerular diseases. 2nd ed. New York: Igaku-shoin; 1995. p. 255–71.
Akikusa B, Sato T, Ogawa M, Ueda S, Kondo Y. Necrotizing alveolar capillaritis in autopsy cases of microscopic polyangitis. Incidence, histopathogenesis, and relationship with systemic vasculitis. Arch Pathol Lab Med. 1997;121:144–9.
Bajema IM, Hagen EC, Ferrario F, Waldherr R, Noel LH, Hermans J, et al. Renal granulomas in systemic vasculitis. Clin Nephrol. 1997;48:16–21.
Cunningham MA, Huang XR, Dowling JP, Tipping PG, Holdsworth SR. Prominence of cell-mediated immunity effectors in “pauci-immune” glomerulonephritis. J Am Soc Nephrol. 1999;10:499–506.
Horio T. Development and fate of crescentic and granulomatous lesions in rat Masugi nephritis. Pathol Int. 2001;51:72–81.
Holdsworth S, Kitching AR, Tipping PG. Th1 and Th2 T helper cell subsets affect patterns of injury and outcomes in glomerulonephritis. Kidney Int. 1999;55:1198–216.
Huang XR, Holdsworth SR, Tipping PG. Evidence for delayed-type hypersensitivity mechanisms in glomerular crescent formation. Kidney Int. 1994;46:69–78.
Tipping PG, Huang XR, Qi M, Van GY, Tang WW. Crescentic glomerulonephritis in CD4- and CD8-deficient mice. Requirement for CD4 but not CD8 cells. Am J Pathol. 1998;152:1541–8.
Huang XR, Tipping PG, Shuo L, Holdsworth SR. Th1 responsiveness to nephritogenic antigens determines susceptibility to crescentic glomerulonephritis in mice. Kidney Int. 1997;51:94–103.
Coelho SN, Saleem S, Konieczny BT, Parekh KR, Baddoura FK, Lakkis FG. Immunologic determinants of susceptibility to experimental glomerulonephritis. Role of cellular immunity. Kidney Int. 1997;51:646–52.
Hopfer H, Maron R, Butzmann U, Helmchen U, Weiner HL, Kalluri R. The importance of cell-mediated immunity in the course and severity of autoimmune anti-glomerular basement membrane disease in mice. FASEB J. 2003;17:860–8.
Kitching AR, Turner AL, Semple T, Li M, Edgtton KL, Wilson GR, et al. Experimental autoimmune anti-glomerular basement membrane glomerulonephritis: a protective role for IFN-γ. J Am Soc Nephrol. 2004;15:1764–74.
Reynolds J, Norgan VA, Bhambra U, Smith J, Cook HT, Pusey CD. Anti-CD8 monoclonal antibody therapy is effective in the prevention and treatment of experimental autoimmune glomerulonephritis. J Am Soc Nephrol. 2002;13:359–69.
Ladel CH, Flesch IEA, Arnoldi J, Kaufmann SHE. Studies with MHC-deficient knock-out mice reveal impact of both MHC I- and MHC II-dependent T cell responses on Listeria monocytogenes infection. J Immunol. 1994;153:3116–22.
Stenger S, Rosat JP, Bloom BR, Krensky AM, Modlin RL. Granulysin: a lethal weapon of cytolytic T cells. Immunol Today. 1999;20:390–4.
Acknowledgments
This work was supported in part by Grant-in-Aid for Scientific Research C-18590524 from the Japan Society for the Promotion of Science (to JM). The authors are grateful to members of the Department of Pathology, Shinshu University School of Medicine, for their encouragement and helpful discussions during this study, and to Ms. Tomoko Nishizawa and Ms. Matsuko Watanabe for their technical assistance.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Aoyagi, D., Nakazawa, K., Kaneyama, T. et al. Granulomatous transformation of capillary lesions in pulmonary-renal syndrome autologously induced anti-glomerular basement membrane disease in Wistar–Kyoto rats. Clin Exp Nephrol 14, 123–131 (2010). https://doi.org/10.1007/s10157-009-0260-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10157-009-0260-9