Abstract
Background
This study investigated the pattern of first recurrence of advanced ovarian cancer before and after the introduction of aggressive surgery.
Methods
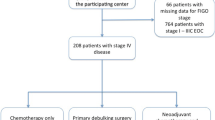
We investigated 291 patients with stage III/IV epithelial ovarian, fallopian tube, and peritoneal cancer. Aggressive surgery including gastrointestinal and upper abdominal surgeries was introduced for advanced ovarian cancer in 2008. The site and time until first recurrence were compared between 70 patients treated without aggressive surgery (2000–2007) and 221 patients who underwent aggressive surgery (2008–2016).
Results
The intraperitoneal recurrence rate was significantly lower in patients treated during 2008–2016 than in patients treated during 2000–2007 (55% [82/149] vs. 81% [46/57], p < 0.001). The median time to intraperitoneal recurrence was significantly longer during 2008–2016 than during 2000–2007 (36.2 months, 95% confidence interval [CI] 31.7–60.0 vs. 14.6 months, 95% CI 11.3–20.1, log-rank test: p < 0.001). However, extraperitoneal recurrence rate was significantly higher during 2008–2016 than during 2000–2007 (27% [40/149] vs. 2% [1/57], p < 0.001). Extraperitoneal recurrence occurred during 2008–2016 in the pleura/lungs and the para-aortic lymph nodes above the renal vessels. Cox proportional hazards regression analysis revealed that treatment period (HR 0.49, 95% CI 0.34–0.71, p < 0.001) and bevacizumab use (HR 0.58, 95% CI 0.39–0.87, p = 0.009) were independently associated with intraperitoneal recurrence; stage IV disease (HR 1.87, 95% CI 1.14–3.06, p = 0.034) was independently associated with extraperitoneal recurrence.
Conclusion
Aggressive surgery reduced intraperitoneal recurrence and prolonged time to recurrence, contributing to better patient survival.


Similar content being viewed by others
References
Chi DS, Eisenhauer EL, Lang J et al (2006) What is the optimal goal of primary cytoreductive surgery for bulky stage IIIC epithelial ovarian carcinoma (EOC)? Gynecol Oncol 103:559–564
Wimberger P, Heubner M, Otterbach F et al (2007) Influence of platinum-based chemotherapy on disseminated tumor cells in blood and bone marrow of patients with ovarian cancer. Gynecol Oncol 107:331–338
Chang SJ, Bristow RE, Ryu HS (2012) Impact of complete cytoreduction leaving no gross residual disease associated with radical cytoreductive surgical procedures on survival in advanced ovarian cancer. Ann Surg Oncol 19:4059–4067
Griffiths CT (1975) Surgical resection of tumor bulk in the primary treatment of ovarian carcinoma. Natl Cancer Inst Monogr 42:101–104
Bristow RE, Tomacruz RS, Armstrong DK et al (2002) Survival effect of maximal cytoreductive surgery for advanced ovarian carcinoma during the platinum era: a meta-analysis. J Clin Oncol 20:1248–1259
du Bois A, Reuss A, Pujade-Lauraine E et al (2009) Role of surgical outcome as prognostic factor in advanced epithelial ovarian cancer: a combined exploratory analysis of 3 prospectively randomized phase 3 multicenter trials: by the Arbeitsgemeinschaft Gynaekologische Onkologie Studiengruppe Ovarialkarzinom (AGO-OVAR) and the Groupe d'Investigateurs Nationaux Pour les Etudes des Cancers de l'Ovaire (GINECO). Cancer 115:1234–1244
Chi DS, Eisenhauer EL, Zivanovic O et al (2009) Improved progression-free and overall survival in advanced ovarian cancer as a result of a change in surgical paradigm. Gynecol Oncol 114:26–31
Tate S, Kato K, Nishikimi K et al (2017) Survival and safety associated with aggressive surgery for stage III/IV epithelial ovarian cancer: a single institution observation study. Gynecol Oncol 147:73–80
Cormio G, Rossi C, Cazzolla A et al (2003) Distant metastases in ovarian carcinoma. Int J Gynecol Cancer 13:125–129
Ferrandina G, Legge F, Salutari V et al (2006) Impact of pattern of recurrence on clinical outcome of ovarian cancer patients: clinical consideration. Eur J Cancer 42:2296–2302
Perri T, Ben-Baruch G, Kalfon G et al (2013) Abdominopelvic cytoreduction rates and recurrence sites in stage IV ovarian cancer: is there a case for thoracic cytoreduction? Gynecol Oncol 131:27–31
Robinson WR, Beyer J, Griffin S et al (2012) Extraperitoneal metastases from recurrent ovarian cancer. Int J Gynecol Cancer 22:43–46
Tanner EJ, Black DR, Zivanovic O et al (2012) Patterns of first recurrence following adjuvant intraperitoneal chemotherapy for stage IIIC ovarian cancer. Gynecol Oncol 124:59–62
Berek JS, Crum C, Friedlander M (2015) Cancer of the ovary, fallopian tube, and peritoneum. Int J Gynaecol Obstet 131:S111–112
Aletti GD, Eisenhauer EL, Santillan A et al (2011) Identification of patient groups at highest risk from traditional approach to ovarian cancer treatment. Gynecol Oncol 120:23–28
Markman M, Markman J, Webster K et al (2004) Duration of response to second-line, platinum-based chemotherapy for ovarian cancer: implications for patient management and clinical trial design. J Clin Oncol 22:3120–3125
Loggie BW, Perini M, Fleming RA et al (1997) Treatment and prevention of malignant ascites associated with disseminated intraperitoneal malignancies by aggressive combined-modality therapy. Am Surg 63:137–143
Malayev Y, Levene R, Gonzalez F (2012) Palliative chemotherapy for malignant ascites secondary to ovarian cancer. Am J Hosp Palliat Care 295:15–21
Petrillo M, Ferrandina G, Fagotti A et al (2013) Timing and pattern of recurrence in ovarian cancer patients with high tumor dissemination treated with primary debulking surgery versus neoadjuvant chemotherapy. Ann Surg Oncol 20:3955–3960
Aletti GD, Podratz KC, Cliby WA et al (2009) Stage IV ovarian cancer: disease site-specific rationale for postoperative treatment. Gynecol Oncol 112:22–27
Esselen KM, Rodriguez N, Growdon W et al (2012) Patterns of recurrence in advanced epithelial ovarian, fallopian tube and peritoneal cancers treated with intraperitoneal chemotherapy. Gynecol Oncol 127:51–54
Usami T, Kato K, Taniguchi T et al (2014) Recurrence patterns of advanced ovarian, fallopian tube, and peritoneal cancers after complete cytoreduction during interval debulking surgery. Int J Gynecol Cancer 24:991–996
Burger RA, Brady MF, Bookman MA et al (2011) Incorporation of bevacizumab in the primary treatment of ovarian cancer. N Engl J Med 365:2473–2483
Perren TJ, Swart AMJ, Pfisterer et al (2011) A phase 3 trial of bevacizumab in ovarian cancer. N Engl J Med 365:2484–2496
Rauh-Hain JA, Guseh SH, Esselen KM et al (2013) Patterns of recurrence in patients treated with bevacizumab in the primary treatment of advanced epithelial ovarian cancer. Int J Gynecol Cancer 23:1219–1225
Dindo D, Demartines N, Clavien PA (2004) Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 240(2):205–213
Chan JK, Brady MF, Penson RT et al (2016) Weekly vs. every-3-week paclitaxel and carboplatin for ovarian cancer. N Engl J Med 374:738–748
Clamp AR, James EC, McNeish IA et al (2019) Weekly dose-dense chemotherapy in first-line epithelial ovarian, fallopian tube, or primary peritoneal carcinoma treatment (ICON8): primary progression free survival analysis results from a GCIG phase 3 randomised controlled trial. Lancet 394:2084–2095
Katsumata N, Yasuda M, Takahashi F et al (2009) Dose-dense paclitaxel once a week in combination with carboplatin every 3 weeks for advanced ovarian cancer: a phase 3, open-label, randomised controlled trial. Lancet 374:1331–1338
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
About this article
Cite this article
Tate, S., Nishikimi, K., Matsuoka, A. et al. Aggressive surgery for advanced ovarian cancer decreases the risk of intraperitoneal recurrence. Int J Clin Oncol 25, 1726–1735 (2020). https://doi.org/10.1007/s10147-020-01714-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-020-01714-w