Abstract
Background
Our specific aim was to investigate the prognostic value of effective duration of first androgen deprivation therapy (ADT) and to evaluate the clinical impact on early docetaxel administration with oncological outcomes in castration-resistant prostate cancer (CRPC) patients treated with docetaxel.
Methods
We identified 148 mCRPC patients who were treated with 75 mg/m2 docetaxel. We defined 16 months as the threshold for the effective duration of ADT, and defined 12 months as the cut-off time for starting docetaxel from the onset of CRPC. Univariate and multivariate analyses were conducted to investigate the prognostic indicators that influenced the survival outcomes.
Results
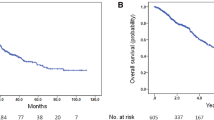
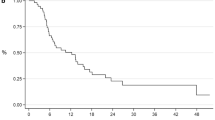
Overall, 81 (54.7%) patients died. The median 1st ADT response was 22.2 months and the median time interval from CRPC onset to docetaxel treatment was 11.7 months. Multivariate analysis indicated that visceral metastasis, bone metastasis extent of disease (EOD) ≥ 2, and effective duration of ADT < 16 months were the independent prognostic indicators for progression-free survival (PFS). Referring to cancer-specific survival (CSS), besides visceral metastasis and effective duration of ADT < 16 months, late docetaxel treatment ≥ 12 months became as the predictors for poor prognosis. Among the ADT poor-responder group (ADT < 16 months), Kaplan–Meier method showed that 1-year and 2-year CSS rates were 96.0% and 80.0% in the patients who introduced docetaxel in early setting (< 12 months), which were significantly higher than those who introduced in late settings (93.6% and 30.8%, respectively, p < 0.001).
Conclusion
CRPC patients who had poor response during 1st ADT would obtain survival benefit by introducing docetaxel treatment in early stage.



Similar content being viewed by others
Change history
02 March 2019
The original article can be found online.
References
Heidenreich A, Bastian PJ, Bellmunt J et al (2014) EAU guidelines on prostate cancer. Part I: screening, diagnosis, and local treatment with curative intent-update 2013. Eur Urol 65:124–137
Heidenreich A, Bastian PJ, Bellmunt J et al (2014) EAU guidelines on prostate cancer. Part II: treatment of advanced, relapsing, and castration-resistant prostate cancer. Eur Urol 65:467–479
Tannock IF, de Wit R, Berry WR et al (2004) Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med 351:1502–1512
Ryan CJ, Smith MR, de Bono JS et al (2013) Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med 368:138–148
Beer TM, Armstrong AJ, Rathkopf DE et al (2014) Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med 371:424–433
de Bono JS, Oudard S, Ozguroglu M et al (2010) Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet 376:1147–1154
Parker C, Nilsson S, Heinrich D et al (2013) Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med 369:213–223
Armstrong AJ, Garrett-Mayer ES, Yang YC et al (2007) A contemporary prognostic nomogram for men with hormone-refractory metastatic prostate cancer: a TAX327 study analysis. Clin Cancer Res 13:6396–6403
Halabi S, Lin CY, Small EJ et al (2013) Prognostic model predicting metastatic castration-resistant prostate cancer survival in men treated with second-line chemotherapy. J Natl Cancer Inst 105:1729–1737
Shigeta K, Kosaka T, Kitano S et al (2016) High absolute monocyte count predicts poor clinical outcome in patients with castration-resistant Prostate cancer treated with docetaxel chemotherapy. Ann Surg Oncol 23:4115–4122
de Bono JS, Scher HI, Montgomery RB et al (2008) Circulating tumor cells predict survival benefit from treatment in metastatic castration-resistant prostate cancer. Clin Cancer Res 14:6302–6309
van Soest RJ, Templeton AJ, Vera-Badillo FE et al (2015) Neutrophil-to-lymphocyte ratio as a prognostic biomarker for men with metastatic castration-resistant prostate cancer receiving first-line chemotherapy: data from two randomized phase III trials. Ann Oncol 26:743–749
Giacinti S, Carlini P, Roberto M et al (2017) Duration of response to first androgen deprivation therapy, time to castration resistance prostate cancer, and outcome of metastatic castration resistance prostate cancer patients treated with abiraterone acetate. Anticancer Drugs 28:110–115
Hongo H, Kosaka T, Mizuno R et al (2016) Should we try antiandrogen withdrawal in castration-resistant prostate cancer patients? Insights from a retrospective study. Clin Genitourin Cancer 14:e569–e573
Scher HI, Morris MJ, Stadler WM et al (2016) Trial design and objectives for castration-resistant prostate cancer: updated recommendations from the Prostate Cancer Clinical Trials Working Group 3. J Clin Oncol 34:1402–1418
Therasse P, Arbuck SG, Eisenhauer EA et al (2000) New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst 92:205–216
Halabi S, Small EJ, Kantoff PW et al (2003) Prognostic model for predicting survival in men with hormone-refractory metastatic prostate cancer. J Clin Oncol 21:1232–1237
Halabi S, Lin CY, Kelly WK et al (2014) Updated prognostic model for predicting overall survival in first-line chemotherapy for patients with metastatic castration-resistant prostate cancer. J Clin Oncol 32:671–677
Suzuki H, Okihara K, Miyake H et al (2008) Alternative nonsteroidal antiandrogen therapy for advanced prostate cancer that relapsed after initial maximum androgen blockade. J Urol 180:921–927
Sartor AO, Tangen CM, Hussain MH et al (2008) Antiandrogen withdrawal in castrate-refractory prostate cancer: a Southwest Oncology Group trial (SWOG 9426). Cancer 112:2393–2400
Kantoff PW, Halabi S, Conaway M et al (1999) Hydrocortisone with or without mitoxantrone in men with hormone-refractory prostate cancer: results of the cancer and leukemia group B 9182 study. J Clin Oncol 17:2506–2513
Sweeney CJ, Chen YH, Carducci M et al (2015) Chemohormonal therapy in metastatic hormone-sensitive prostate cancer. N Engl J Med 373:737–746
Vale CL, Burdett S, Rydzewska LH et al (2016) Addition of docetaxel or bisphosphonates to standard of care in men with localised or metastatic, hormone-sensitive prostate cancer: a systematic review and meta-analyses of aggregate data. Lancet Oncol 17:243–256
Bracarda S, Caserta C, Galli L et al (2015) Docetaxel rechallenge in metastatic castration-resistant prostate cancer: any place in the modern treatment scenario? An intention to treat evaluation. Future Oncol 11:3083–3090
Sonpavde G, Huang A, Wang L et al (2018) Taxane chemotherapy vs antiandrogen agents as first-line therapy for metastatic castration-resistant prostate cancer. BJU Int 121:871–879
Acknowledgements
This work was supported in part by Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (#17K11158) and the Prostate Research Fund in Japan. The study was supported in part by research Grants to T. Kosaka from the Takeda Science Foundation, Japan, the Japan Research Foundation for Clinical Pharmacology and the Yamaguchi Endocrine Research Foundation. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None of the authors has any conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original version of this article was revised: In the Original Publication, Tables 2 and 3 have been published with “###” instead of numerical values. Also in Table 2, the bold entries are made unbold. The errors have been occurred at Publishers end. The tables have been corrected.
Electronic supplementary material
Below is the link to the electronic supplementary material.
About this article
Cite this article
Shigeta, K., Kosaka, T., Hongo, H. et al. Castration-resistant prostate cancer patients who had poor response on first androgen deprivation therapy would obtain certain clinical benefit from early docetaxel administration. Int J Clin Oncol 24, 546–553 (2019). https://doi.org/10.1007/s10147-018-01388-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-018-01388-5