Abstract
Background
It is known that one third of patients with human epidermal growth factor receptor 2 (HER2)-overexpressing metastatic breast cancer (MBC) treated with trastuzumab will develop brain metastases. As the development of brain metastases is fatal, controlling its progression is clinically meaningful. However, effective therapy for MBC patients with brain metastasis after cranial radiation is limited. The international clinical study in which six Japanese patients participated indicated the antitumor activity of lapatinib against brain metastases of HER2-overexpressing breast cancer.
Methods
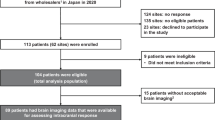
The efficacy, safety, and pharmacokinetics of lapatinib 750 mg given twice daily to Japanese HER2-overexpressing MBC patients with brain metastases were assessed as part of the international clinical study.
Results
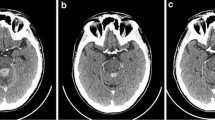
Of six Japanese patients treated in this study, two patients had shown volumetric reduction >20 % in their central nervous system (CNS), one of whom had >50 % reduction. Three patients, including two of these patients, had shown >20 % volumetric reduction in non-CNS lesions. Frequently reported adverse events were diarrhea and rash, all of which were controllable. The AUC0–12 of lapatinib on day 28 was 1.74 times higher than that on day 1.
Conclusion
These results suggest that lapatinib monotherapy 750 mg given twice daily can exert some efficacy and has potential as a clinically meaningful treatment option for Japanese HER2-overexpressing breast cancer patients with brain metastases after cranial radiation.



Similar content being viewed by others
References
Burstein HJ, Lieberman G, Slamon DJ et al (2005) Isolated central nervous system metastases in patients with HER2-overexpressing advance breast cancer treated with first-line trastuzumab-based therapy. Ann Oncol 16:1772–1777
Pestalozzi BC, Zahrieh D, Price KN et al (2006) Identifying breast cancer patients at risk for central nervous system (CNS) metastases in trials of the International Breast Cancer Study Group (IBCSG). Ann Oncol 17:935–944
Bendell JC, Domchek SM, Burstein HJ et al (2003) Central nervous system metastases in women who receive trastuzumab-based therapy for metastatic breast carcinoma. Cancer (Phila) 97:2972–2977
Clayton AJ, Danson S, Jolly S et al (2004) Incidence of cerebral metastases in patients treated with trastuzumab for metastatic breast cancer. Br J Cancer 91:639–643
Lin NU, Winer EP (2007) Brain metastases: the HER2 paradigm. Clin Cancer Res 13:1648–1655
Stemmler HJ, Kahlert S, Siekiera W et al (2006) Characteristics of patients with brain metastases receiving trastuzumab for HER2 overexpressing metastatic breast cancer. Breast 15:219–225
Yau T, Swanton C, Chua S et al (2006) Incidence, pattern and timing of brain metastases among patients with advanced breast cancer treated with trastuzumab. Acta Oncol 45:196–201
Toyama T (2009) 7. Lapatinib and brain metastasis, clinical and experimental aspects of breast cancer. In: Toi M (ed) Iyaku (Medicine and Drug). Journal Co., Tokyo, p 877
Bezjak A, Adam J, Barton R et al (2002) Symptom response after palliative radiotherapy for patients with brain metastases. Eur J Cancer 38:487–496
Arslan C, Dizdar O, Altundag K (2010) Systemic treatment in breast-cancer patients with brain metastasis. Expert Opin Pharmacother 11:1089–1100
Rusnak DW, Lackey K, Affleck K et al (2001) The effects of the novel, reversible epidermal growth factor receptor/ErbB-2 tyrosine kinase inhibitor, GW2016, on the growth of human normal and tumor-derived cell lines in vitro and in vivo. Mol Cancer Ther 1:85–94
Gril B, Palmieri D, Bronder JL et al (2008) Effect of lapatinib on the outgrowth of metastatic breast cancer cells to the brain. J Natl Cancer Inst 100:1092–1103
Lin NU, Carey LA, Liu MC et al (2008) Phase II trial of lapatinib for brain metastases in patients with human epidermal growth factor receptor 2-positive breast cancer. J Clin Oncol 26:1993–1999
Lin NU, Dieras V, Paul D et al (2009) Multicenter phase II study of lapatinib in patients with brain metastases from HER2-positive breast cancer. Clin Cancer Res 15:1452–1459
Nakagawa K, Minami H, Kanezaki M et al (2009) Phase I dose-escalation and pharmacokinetic trial of lapatinib (GW572016), a selective oral dual inhibitor of ErbB-1 and -2 tyrosine kinases, in Japanese patients with solid tumors. Jpn J Clin Oncol 39:116–123
Toi M, Iwata H, Fujiwara Y et al (2009) Lapatinib monotherapy in patients with relapsed, advanced, or metastatic breast cancer: efficacy, safety, and biomarker results from Japanese patients phase II studies. Br J Cancer 101:1676–1682
Burstein HJ, Storniolo AM, Franco S et al (2008) A phase II study of lapatinib monotherapy in chemotherapy-refractory HER2-positive and HER2-negative advanced or metastatic breast cancer. Ann Oncol 19:1068–1074
Blackwell KL, Pegram MD, Tan-Chiu E et al (2009) Single-agent lapatinib for the treatment of HER2-overexpressing advanced or metastatic breast cancer that progressed on first- or second-line trastuzumab containing regimens. Ann Oncol 20:1026–1031
Burris HA III, Hurwitz HI, Dees EC et al (2005) Phase I safety, pharmacokinetics, and clinical activity study of lapatinib (GW572016), a reversible dual inhibitor of epidermal growth factor receptor tyrosine kinases, in heavily pretreated patients with metastatic carcinomas. J Clin Oncol 23:5305–5313
Burris HA III, Taylor CW, Jones SF et al (2009) A phase I and pharmacokinetic study of oral lapatinib administered once or twice daily in patients with solid malignancies. Clin Cancer Res 15:6702–6708
Stemmler HJ, Schmitt M, Willems A et al (2007) Ratio of trastuzumab levels in serum and cerebrospinal fluid is altered in HER2-positive breast cancer patients with brain metastases and impairment of blood–brain barrier. Anticancer Drugs 18:23–28
Acknowledgments
We thank the women who participated in these studies and their families. The authors also acknowledge the dedicated work of all the research nurses and study coordinators at the study centers. This work was sponsored by GlaxoSmithKline K.K.
Conflict of interest
All authors have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Iwata, H., Narabayashi, M., Ito, Y. et al. A phase II study of lapatinib for brain metastases in patients with HER2-overexpressing breast cancer following trastuzumab based systemic therapy and cranial radiotherapy: subset analysis of Japanese patients. Int J Clin Oncol 18, 621–628 (2013). https://doi.org/10.1007/s10147-012-0444-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-012-0444-2