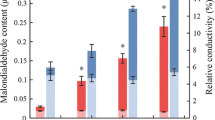
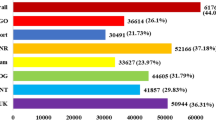
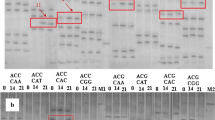
Abstract
Aegilops tauschii is the diploid progenitor of the bread wheat D-genome. It originated from Iran and is a source of abiotic stress tolerance genes. However, little is known about the molecular events of salinity tolerance in Ae. tauschii. This study investigates the leaf transcriptional changes associated with long-term salt stress. Total RNA extracted from leaf tissues of control and salt-treated samples was sequenced using the Illumina technology, and more than 98 million high-quality reads were assembled into 255,446 unigenes with an average length of 1398 bp and an N50 of 2269 bp. Functional annotation of the unigenes showed that 93,742 (36.69%) had at least a significant BLAST hit in the SwissProt database, while 174,079 (68.14%) showed significant similarity to proteins in the NCBI nr database. Differential expression analysis identified 4506 salt stress-responsive unigenes. Bioinformatic analysis of the differentially expressed unigenes (DEUs) revealed a number of biological processes and pathways involved in the establishment of ion homeostasis, signaling processes, carbohydrate metabolism, and post-translational modifications. Fine regulation of starch and sucrose content may be important features involved in salt tolerance in Ae. tauschii. Moreover, 82% of DEUs mapped to the D-subgenome, including known QTL for salt tolerance, and these DEUs showed similar salt stress responses in other accessions of Ae. tauschii. These results could provide fundamental insight into the regulatory process underlying salt tolerance in Ae. tauschii and wheat and facilitate identification of genes involved in their salt tolerance mechanisms.








Similar content being viewed by others
Abbreviations
- DEUs:
-
Differentially expressed unigenes
- Na:
-
Sodium
- Cl:
-
Chlorine
- K:
-
Potassium
- BLAST:
-
Basic local alignment search tool
- nr :
-
Non-redundant
- qRT-PCR:
-
Quantitative real-time PCR
References
Alptekin B, Budak H (2017) Wheat miRNA ancestors: evident by transcriptome analysis of A, B, and D genome donors. Funct Integr Genomics 17:171–187
Ashraf M, Öztürk MA, Athar H (2008) Salinity and water stress: improving crop efficiency vol 44. Springer Science & Business Media,
Baena-González E, Rolland F, Thevelein JM, Sheen J (2007) A central integrator of transcription networks in plant stress and energy signalling. Nature 448:938–942
Balibrea ME, Dell'Amico J, Bolarín MC, Pérez-Alfocea F (2000) Carbon partitioning and sucrose metabolism in tomato plants growing under salinity. Physiol Plant 110:503–511
Blumwald E, Aharon GS, Apse MP (2000) Sodium transport in plant cells. Biochim Biophys Acta (BBA)-Biomembr 1465:140–151
Bolger AM, Lohse M, Usadel B (2014) Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics:btu170
Boudsocq M, Sheen J (2013) CDPKs in immune and stress signaling. Trends Plant Sci 18:30–40
Brummell DA, Chen RKY, Harris JC, Zhang H, Hamiaux C, Kralicek AV, McKenzie MJ (2011) Induction of vacuolar invertase inhibitor mRNA in potato tubers contributes to cold-induced sweetening resistance and includes spliced hybrid mRNA variants. J Exp Bot 62:3519–3534. https://doi.org/10.1093/jxb/err043
Capitão C, Paiva JA, Santos DM, Fevereiro P (2011) In Medicago truncatula, water deficit modulates the transcript accumulation of components of small RNA pathways. BMC Plant Biol 11(1)
Chen Z, Hong X, Zhang H, Wang Y, Li X, Zhu JK, Gong Z (2005) Disruption of the cellulose synthase gene, AtCesA8/IRX1, enhances drought and osmotic stress tolerance in Arabidopsis. Plant J 43:273–283
Cheng D, Wu G, Zheng Y (2015) Positive correlation between potassium uptake and salt tolerance in wheat. Photosynthetica 53:447–454
Colmer T, Munns R, Flowers T (2005) Improving salt tolerance of wheat and barley: future prospects. Anim Prod Sci 45:1425–1443
Colmer TD, Flowers TJ, Munns R (2006) Use of wild relatives to improve salt tolerance in wheat. J Exp Bot 57:1059–1078
Conesa A, Götz S, García-Gómez JM, Terol J, Talón M, Robles M (2005) Blast2GO: a universal tool for annotation, visualization and analysis in functional genomics research. Bioinformatics 21:3674–3676
Cui MH et al (2013) An Arabidopsis R2R3-MYB transcription factor, AtMYB20, negatively regulates type 2C serine/threonine protein phosphatases to enhance salt tolerance. FEBS Lett 587:1773–1778
Deinlein U, Stephan AB, Horie T, Luo W, Xu G, Schroeder JI (2014) Plant salt-tolerance mechanisms. Trends Plant Sci 19:371–379
Du Z, Zhou X, Ling Y, Zhang Z, Su Z (2010) agriGO: a GO analysis toolkit for the agricultural community Nucleic acids research:gkq310
Galon Y, Nave R, Boyce JM, Nachmias D, Knight MR, Fromm H (2008) Calmodulin-binding transcription activator (CAMTA) 3 mediates biotic defense responses in Arabidopsis. FEBS Lett 582:943–948
Gaxiola RA, Li J, Undurraga S, Dang LM, Allen GJ, Alper SL, Fink GR (2001) Drought-and salt-tolerant plants result from overexpression of the AVP1 H+−pump. Proc Natl Acad Sci 98:11444–11449
Goetz M, Godt DE, Guivarc'h A, Kahmann U, Chriqui D, Roitsch T (2001) Induction of male sterility in plants by metabolic engineering of the carbohydrate supply. Proc Natl Acad Sci 98:6522–6527
Golldack D, Lüking I, Yang O (2011) Plant tolerance to drought and salinity: stress regulating transcription factors and their functional significance in the cellular transcriptional network. Plant Cell Rep 30:1383–1391
Gorham J, Hardy C, Jones RW, Joppa L, Law C (1987) Chromosomal location of a K/Na discrimination character in the D genome of wheat. Theor Appl Genet 74:584–588
Grabherr MG et al (2011) Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat Biotechnol 29:644–652
Guo A-Y et al (2008) PlantTFDB: a comprehensive plant transcription factor database. Nucleic Acids Res 36:D966–D969
Gupta B, Huang B (2014, 2014) Mechanism of salinity tolerance in plants: physiological, biochemical, and molecular characterization. Int J Genomics
Halfter U, Ishitani M, Zhu J-K (2000) The Arabidopsis SOS2 protein kinase physically interacts with and is activated by the calcium-binding protein SOS3. Proc Natl Acad Sci 97:3735–3740
Harmon AC, Gribskov M, Harper JF (2000) CDPKs—a kinase for every Ca 2+ signal? Trends Plant Sci 5:154–159
Horie T et al (2005) Enhanced salt tolerance mediated by AtHKT1 transporter-induced Na+ unloading from xylem vessels to xylem parenchyma cells. Plant J 44:928–938
Horie T et al (2011) Rice sodium-insensitive potassium transporter, OsHAK5, confers increased salt tolerance in tobacco BY2 cells. J Biosci Bioeng 111:346–356
Hussain B, Lucas SJ, Ozturk L, Budak H (2017) Mapping QTLs conferring salt tolerance and micronutrient concentrations at seedling stage in wheat. Sci Rep 7:15662
Ishitani M, Liu J, Halfter U, Kim C-S, Shi W, Zhu J-K (2000) SOS3 function in plant salt tolerance requires N-myristoylation and calcium binding. Plant Cell 12:1667–1677
James RA, Blake C, Byrt CS, Munns R (2011) Major genes for Na+ exclusion, Nax1 and Nax2 (wheat HKT1; 4 and HKT1; 5), decrease Na+ accumulation in bread wheat leaves under saline and waterlogged conditions. J Exp Bot 62:2939–2947
Jaspers P, Kangasjärvi J (2010) Reactive oxygen species in abiotic stress signaling. Physiol Plant 138:405–413
Jia J et al (2013) Aegilops tauschii draft genome sequence reveals a gene repertoire for wheat adaptation. Nature 496:91–95
Jiang Y, Deyholos MK (2009) Functional characterization of Arabidopsis NaCl-inducible WRKY25 and WRKY33 transcription factors in abiotic stresses. Plant Mol Biol 69:91–105
Jiang Y, Yang B, Deyholos MK (2009) Functional characterization of the Arabidopsis bHLH92 transcription factor in abiotic stress. Mol Gen Genomics 282:503–516
Jiang Z, Zhu S, Ye R, Xue Y, Chen A, An L, Pei Z-M (2013) Relationship between NaCl-and H 2 O 2-induced cytosolic Ca 2+ increases in response to stress in Arabidopsis. PLoS One 8
Kao W-Y, Tsai T-T, Tsai H-C, Shih C-N (2006) Response of three Glycine species to salt stress. Environ Exp Bot 56:120–125
Kasuga M, Liu Q, Miura S, Yamaguchi-Shinozaki K, Shinozaki K (1999) Improving plant drought, salt, and freezing tolerance by gene transfer of a single stress-inducible transcription factor. Nat Biotechnol 17:287–291
Kawaura K, Mochida K, Ogihara Y (2008) Genome-wide analysis for identification of salt-responsive genes in common wheat. Functional & integrative genomics 8:277–286
Krapp A, Stitt M (1995) An evaluation of direct and indirect mechanisms for the “sink-regulation” of photosynthesis in spinach: changes in gas exchange, carbohydrates, metabolites, enzyme activities and steady-state transcript levels after cold-girdling source leaves. Planta 195:313–323
Langmead B, Trapnell C, Pop M, Salzberg SL (2009) Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol 10:R25
Liu P, Yang G-D, Li H, Zheng C-C, Wu C-A (2010) Overexpression of NHX1s in transgenic Arabidopsis enhances photoprotection capacity in high salinity and drought conditions. Acta Physiol Plant 32:81–90
Liu J et al (2015a) Down-regulation of a wheat alkaline/neutral invertase correlates with reduced host susceptibility to wheat stripe rust caused by Puccinia striiformis. J Exp Bot 66:7325–7338
Liu Y et al (2015b) Genome-wide association study of 29 morphological traits in Aegilops tauschii. Sci Rep 5:15562
Lohse M et al (2014) Mercator: a fast and simple web server for genome scale functional annotation of plant sequence data. Plant Cell Environ 37:1250–1258
Long X-Y et al (2010) Genome-wide identification and evaluation of novel internal control genes for Q-PCR based transcript normalization in wheat. Plant Mol Biol 74:307–311
Luo M-C et al (2017) Genome sequence of the progenitor of the wheat D genome Aegilops tauschii. Nature 551
Ma S, Niu H, Liu C, Zhang J, Hou C, Wang D (2013) Expression stabilities of candidate reference genes for RT-qPCR under different stress conditions in soybean. PLoS One 8:e75271
Mao X, Cai T, Olyarchuk JG, Wei L (2005) Automated genome annotation and pathway identification using the KEGG Orthology (KO) as a controlled vocabulary. Bioinformatics 21:3787–3793
Mazzucotelli E, Mastrangelo AM, Crosatti C, Guerra D, Stanca AM, Cattivelli L (2008) Abiotic stress response in plants: when post-transcriptional and post-translational regulations control transcription. Plant Sci 174:420–431
Mi S et al (2008) Sorting of small RNAs into Arabidopsis argonaute complexes is directed by the 5′ terminal nucleotide. Cell 133:116–127
Miller G, Suzuki N, CIFTCI-YILMAZ S, Mittler R (2010) Reactive oxygen species homeostasis and signalling during drought and salinity stresses. Plant, Cell Environ 33:453–467
Moriya Y, Itoh M, Okuda S, Yoshizawa AC, Kanehisa M (2007) KAAS: an automatic genome annotation and pathway reconstruction server. Nucleic Acids Res 35:W182–W185
Munnik T, Meijer HJ (2001) Osmotic stress activates distinct lipid and MAPK signalling pathways in plants. FEBS Lett 498:172–178
Munns R (2013) Hoagland’s nutrient solution. csiro
Munns R, James RA (2003) Screening methods for salinity tolerance: a case study with tetraploid wheat. Plant Soil 253:201–218
Munns R, Tester M (2008) Mechanisms of salinity tolerance. Annu Rev Plant Biol 59:651–681
Naghavi MR, Mardi M (2010) Characterization of genetic variation among accessions of Aegilops tauschii. AsPac J Mol Biol Biotechno 18:93–96
Nakashima K, Takasaki H, Mizoi J, Shinozaki K, Yamaguchi-Shinozaki K (2012) NAC transcription factors in plant abiotic stress responses. Biochim Biophys Acta (BBA)-Gene Regul Mech 1819:97–103
Nevo E (2005) Genomic diversity in nature and domestication. In: Henry R (ed) Plant diversity and evolution: genotypic and phenotypic variation in higher plants. CABI publishing CAB International, Wallingford, U.K, pp 287–316
Nevo E, Chen G (2010) Drought and salt tolerances in wild relatives for wheat and barley improvement. Plant Cell Environ 33:670–685
Oyiga BC, Sharma RC, Baum M, Ogbonnaya FC, Léon J, Ballvora A (2017) Allelic variations and differential expressions detected at quantitative trait loci for salt stress tolerance in wheat. Plant, Cell Environ
Pandey N et al (2013) CAMTA 1 regulates drought responses in Arabidopsis thaliana. BMC Genomics 14:216
Pfaffl MW, Horgan GW, Dempfle L (2002) Relative expression software tool (REST©) for group-wise comparison and statistical analysis of relative expression results in real-time. PCR. Nucleic Acids Res 30:e36–e36
Pritchard D, Hollington P, Davies W, Gorham J, de Leon JD, Mujeeb-Kazi A (2002) K+/Na+ discrimination in synthetic hexaploid wheat lines: transfer of the trait for K+/Na+ discrimination from Aegilops tauschii into a Triticum turgidum background. Cereal Res Commun:261–267
Qian Y, Cheng Y, Cheng X, Jiang H, Zhu S, Cheng B (2011) Identification and characterization of Dicer-like, Argonaute and RNA-dependent RNA polymerase gene families in maize. Plant Cell Rep 30:1347–1363
Qiu Q-S, Guo Y, Dietrich MA, Schumaker KS, Zhu J-K (2002) Regulation of SOS1, a plasma membrane Na+/H+ exchanger in Arabidopsis thaliana, by SOS2 and SOS3. Proc Natl Acad Sci 99:8436–8441
Quarrie S et al (2005) A high-density genetic map of hexaploid wheat (Triticum aestivum L.) from the cross Chinese Spring× SQ1 and its use to compare QTLs for grain yield across a range of environments. Theor Appl Genet 110:865–880
Quintero FJ, Ohta M, Shi H, Zhu J-K, Pardo JM (2002) Reconstitution in yeast of the Arabidopsis SOS signaling pathway for Na+ homeostasis. Proceedings of the Natl Acad Sci 99:9061–9066
Rahaie M, Xue G-P, Schenk PM (2013) The role of transcription factors in wheat under different abiotic stresses. In: Abiotic stress-plant responses and applications in agriculture. InTech
Riaño-Pachón DM, Ruzicic S, Dreyer I, Mueller-Roeber B (2007) PlnTFDB: an integrative plant transcription factor database. BMC Bioinf 8(42)
Roitsch T, González M-C (2004) Function and regulation of plant invertases: sweet sensations. Trends Plant Sci 9:606–613
Roitsch T, Balibrea M, Hofmann M, Proels R, Sinha A (2003) Extracellular invertase: key metabolic enzyme and PR protein. J Exp Bot 54:513–524
Sanchez DH et al (2008) Integrative functional genomics of salt acclimatization in the model legume Lotus japonicus. Plant J 53:973–987
Sathee L, Sairam RK, Chinnusamy V, Jha SK (2015) Differential transcript abundance of salt overly sensitive (SOS) pathway genes is a determinant of salinity stress tolerance of wheat. Acta Physiol Plant 37:169
Schachtman D, Munns R, Whitecross M (1991) Variation in sodium exclusion and salt tolerance in Triticum tauschii. Crop Sci 31:992–997
Schachtman D, Lagudah E, Munns R (1992) The expression of salt tolerance from Triticum tauschii in hexaploid wheat. Theor Appl Genet 84:714–719
Shi H, Quintero FJ, Pardo JM, Zhu J-K (2002) The putative plasma membrane Na+/H+ antiporter SOS1 controls long-distance Na+ transport in plants. Plant Cell 14:465–477
Sohail Q, Inoue T, Tanaka H, Eltayeb AE, Matsuoka Y, Tsujimoto H (2011) Applicability of Aegilops tauschii drought tolerance traits to breeding of hexaploid wheat. Breed Sci 61:347
Thimm O et al (2004) MapMan: a user-driven tool to display genomics data sets onto diagrams of metabolic pathways and other biological processes. Plant J 37:914–939
Tran L-SP et al (2004) Isolation and functional analysis of Arabidopsis stress-inducible NAC transcription factors that bind to a drought-responsive cis-element in the early responsive to dehydration stress 1 promoter. Plant Cell 16:2481–2498
Tran L-SP, Nishiyama R, Yamaguchi-Shinozaki K, Shinozaki K (2010) Potential utilization of NAC transcription factors to enhance abiotic stress tolerance in plants by biotechnological approach. GM Crops 1:32–39
Wang B, Lüttge U, Ratajczak R (2001) Effects of salt treatment and osmotic stress on V-ATPase and V-PPase in leaves of the halophyte Suaeda salsa. J Exp Bot 52:2355–2365
Wang S et al (2014) Characterization of polyploid wheat genomic diversity using a high-density 90 000 single nucleotide polymorphism array. Plant Biotechnol J 12:787–796
Weinl S, Kudla J (2009) The CBL–CIPK Ca2+−decoding signaling network: function and perspectives. New Phytol 184:517–528
Weschke W, Panitz R, Gubatz S, Wang Q, Radchuk R, Weber H, Wobus U (2003) The role of invertases and hexose transporters in controlling sugar ratios in maternal and filial tissues of barley caryopses during early development. Plant J 33:395–411
Witcombe JR, Hollington PA, Howarth CJ, Reader S, Steele KA (2008) Breeding for abiotic stresses for sustainable agriculture. Philos Trans R Soc B: Biol Sci 363:703–716. https://doi.org/10.1098/rstb.2007.2179
Xie W, Nevo E (2008) Wild emmer: genetic resources, gene mapping and potential for wheat improvement. Euphytica 164:603–614
Xiong L, Schumaker KS, Zhu J-K (2002) Cell signaling during cold, drought, and salt stress. Plant Cell 14:S165–S183
Yang T, Poovaiah B (2002) A calmodulin-binding/CGCG box DNA-binding protein family involved in multiple signaling pathways in plants. J Biol Chem 277:45049–45058
Yang O, Popova OV, Süthoff U, Lüking I, Dietz K-J, Golldack D (2009) The Arabidopsis basic leucine zipper transcription factor AtbZIP24 regulates complex transcriptional networks involved in abiotic stress resistance. Gene 436:45–55
Yoo JH et al (2005) Direct interaction of a divergent CaM isoform and the transcription factor, MYB2, enhances salt tolerance in Arabidopsis. J Biol Chem 280:3697–3706
Zhu J-K (2001) Plant salt tolerance. Trends Plant Sci 6:66–71
Zhu J-K (2002) Salt and drought stress signal transduction in plants. Annu Rev Plant Biol 53:247
Zhu J-K (2003) Regulation of ion homeostasis under salt stress. Curr Opin Plant Biol 6:441–445
Funding
This study was supported by a grant from the Iran National Foundation Science (project number: 90003984).
Author information
Authors and Affiliations
Contributions
MM, MRN, HA, and GM-N conceived and designed the research. MM and SAM performed the experiments and analyzed the data. MM, MRN, HA, SAM, and YT interpreted the results and wrote the manuscript. MRN, HA, GM-N, and GHS supervised the study. All authors read and approved the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
ESM 1
(XLSX 2432 kb)
Rights and permissions
About this article
Cite this article
Mansouri, M., Naghavi, M.R., Alizadeh, H. et al. Transcriptomic analysis of Aegilops tauschii during long-term salinity stress. Funct Integr Genomics 19, 13–28 (2019). https://doi.org/10.1007/s10142-018-0623-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10142-018-0623-y