Abstract
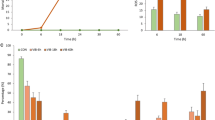
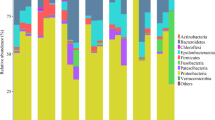
Vibrio coralliilyticus is a bacterial pathogen which can affect a range of marine organisms, such as corals, fish and shellfish, with sometimes devastating consequences. However, little is known about the mechanisms involved in the host-pathogen interaction, especially within molluscan models. We applied gas chromatography-mass spectrometry (GC-MS)-based metabolomics to characterize the physiological responses in haemolymph of New Zealand Greenshell™ mussels (Perna canaliculus) injected with Vibrio sp. DO1 (V. coralliilyticus/neptunius-like isolate). Univariate data analyses of metabolite profiles in Vibrio-exposed mussels revealed significant changes in 22 metabolites at 6 h post-infection, compared to non-exposed mussels. Among them, 10 metabolites were up-regulated, while 12 metabolites were down-regulated in infected mussels. Multivariate analyses showed a clear distinction between infected and non-infected mussels. In addition, secondary pathway analyses indicated perturbations of the host innate immune system following infection, including oxidative stress, inflammation and disruption of the TCA cycle, change in amino acid metabolism and protein synthesis. These findings provide new insights into the pathogenic mechanisms of Vibrio infection of mussels and demonstrate our ability to detect detailed and rapid host responses from haemolymph samples using a metabolomics approach.





Similar content being viewed by others
References
Alfaro AC, Young T (2016) Showcasing metabolomic applications in aquaculture: a review. Rev Aquac. https://doi.org/10.1111/raq.12152
Allam B, Raftos D (2015) Immune responses to infectious diseases in bivalves. J Invertebr Pathol 131:121–136
Anderson RS, Good RA (1976) Opsonic involvement in phagocytosis by mollusk hemocytes. J Invertebr Pathol 27:57–64
Asensi M, Sastre J, Pallardo FV, Lloret A, Lehner M, Garcia-de-la Asuncion J, Viña J (1999) [23] ratio of reduced to oxidized glutathione as indicator of oxidative stress status and DNA damage. Methods Enzymol 299:267–276
Austin B, Austin D, Sutherland R, Thompson F, Swings J (2005) Pathogenicity of vibrios to rainbow trout (Oncorhynchus mykiss, Walbaum) and Artemia nauplii. Environ Microbiol 7:1488–1495
Bachère E, Rosa RD, Schmitt P, Poirier AC, Merou N, Charrière GM, Destoumieux-Garzón D (2015) The new insights into the oyster antimicrobial defense: cellular, molecular and genetic view. Fish Shellfish Immunol 46:50–64
Bai R, You W, Chen J, Huang H, Ke C (2012) Molecular cloning and expression analysis of GABA a receptor-associated protein (GABARAP) from small abalone, Haliotis diversicolor. Fish Shellfish Immunol 33:675–682
Bassim S, Genard B, Gauthier-Clerc S, Moraga D, Tremblay R (2015) Ontogeny of bivalve immunity: assessing the potential of next-generation sequencing techniques. Rev Aquac 7:197–217
Ben-Haim Y, Thompson FL, Thompson CC, Cnockaert MC, Hoste B, Swings J, Rosenberg E (2003) Vibrio coralliilyticus sp. nov., a temperature-dependent pathogen of the coral Pocillopora damicornis. Int J Syst Evol Microbiol 53:309–315
Bindokas VP, Jordan J, Lee CC, Miller RJ (1996) Superoxide production in rat hippocampal neurons: selective imaging with hydroethidine. J Neurosci 16:1324–1336
Brown C (1981) A study of two shellfish-pathogenic Vibrio strains isolated from a Long Island hatchery during a recent outbreak of disease [New York]. J Shellfish Res 1:83–87
Buggé DM, Hégaret H, Wikfors GH, Allam B (2007) Oxidative burst in hard clam (Mercenaria mercenaria) haemocytes. Fish Shellfish Immunol 23:188–196
Calder PC (2006) Branched-chain amino acids and immunity. J Nutr 136:288S–293S
Choi SH, Jee BY, Lee SJ, Cho MY, Lee SJ, Kim JW, Jeong HD, Kim KH (2013) Effects of RNA interference-mediated knock-down of hypoxia-inducible factor-α on respiratory burst activity of the Pacific oyster Crassostrea gigas hemocytes. Fish Shellfish Immunol 35:476–479
Chouchani ET, Pell VR, Gaude E, Aksentijević D, Sundier SY, Robb EL, Logan A, Nadtochiy SM, Ord ENJ, Smith AC, Eyassu F, Shirley R, Hu CH, Dare AJ, James AM, Rogatti S, Hartley RC, Eaton S, Costa ASH, Brookes PS, Davidson SM, Duchen MR, Saeb-Parsy K, Shattock MJ, Robinson AJ, Work LM, Frezza C, Krieg T, Murphy MP (2014) Ischaemic accumulation of succinate controls reperfusion injury through mitochondrial ROS. Nature 515:431–435
Costa MM, Prado-Alvarez M, Gestal C, Li H, Roch P, Novoa B, Figueras A (2009) Functional and molecular immune response of Mediterranean mussel (Mytilus galloprovincialis) haemocytes against pathogen-associated molecular patterns and bacteria. Fish Shellfish Immunol 26:515–523
De Vico G, Carella F (2012) Morphological features of the inflammatory response in molluscs. Res Vet Sci 93:1109–1115
Dumbauld BR, Kauffman BE, Trimble AC, Ruesink JL (2011) The Willapa Bay oyster reserves in Washington state: fishery collapse, creating a sustainable replacement, and the potential for habitat conservation and restoration. J Shellfish Res 30:71–83
Elston R, Leibovitz L, Relyea D, Zatila J (1981) Diagnosis of vibriosis in a commercial oyster hatchery epizootic: diagnostic tools and management features. Aquaculture 24:53–62
Elston RA, Hasegawa H, Humphrey KL, Polyak IK, Häse CC (2008) Re-emergence of vibrio tubiashii in bivalve shellfish aquaculture: severity, environmental drivers, geographic extent and management. Dis Aquat Org 82:119–134
Eriksson KS, Panula P (1994) Gamma-aminobutyric acid in the nervous system of a planarian. J Comp Neurol 345:528–536
Espinosa-Diez C, Miguel V, Mennerich D, Kietzmann T, Sánchez-Pérez P, Cadenas S, Lamas S (2015) Antioxidant responses and cellular adjustments to oxidative stress. Redox Biol 6:183–197
Estes RM, Friedman CS, Elston RA, Herwig RP (2004) Pathogenicity testing of shellfish hatchery bacterial isolates on Pacific oyster Crassostrea gigas larvae. Dis Aquat Org 58:223–230
Fleury E, Huvet A, Lelong C, de Lorgeril J, Boulo V, Gueguen Y, Bachère E, Tanguy A, Moraga D, Fabioux C, Lindeque P, Shaw J, Reinhardt R, Prunet P, Davey G, Lapègue S, Sauvage C, Corporeau C, Moal J, Gavory F, Wincker P, Moreews F, Klopp C, Mathieu M, Boudry P, Favrel P (2009) Generation and analysis of a 29,745 unique expressed sequence tags from the Pacific oyster (Crassostrea gigas) assembled into a publicly accessible database: the GigasDatabase. BMC Genomics 10:341
Fubini B, Hubbard A (2003) Reactive oxygen species (ROS) and reactive nitrogen species (RNS) generation by silica in inflammation and fibrosis. Free Radic Biol Med 34:1507–1516
Gaber T, Strehl C, Buttgereit F (2017) Metabolic regulation of inflammation. Nat Rev Rheumatol 13:267–279
Genard B, Miner P, Nicolas J-L, Moraga D, Boudry P, Pernet F, Tremblay R (2013) Integrative study of physiological changes associated with bacterial infection in Pacific oyster larvae. PLoS One 8:e64534
Goedken M, De Guise S (2004) Flow cytometry as a tool to quantify oyster defence mechanisms. Fish Shellfish Immunol 16:539–552
Gómez-Chiarri M, Guo X, Tanguy A, He Y, Proestou D (2015) The use of -omic tools in the study of disease processes in marine bivalve mollusks. J Invertebr Pathol 131:137–154
Grandiosa R, Mérien F, Pillay K, Alfaro A (2016) Innovative application of classic and newer techniques for the characterization of haemocytes in the New Zealand black-footed abalone (Haliotis iris). Fish Shellfish Immunol 48:175–184
Guo X, Ford SE (2016) Infectious diseases of marine molluscs and host responses as revealed by genomic tools. Philos Trans R Soc B 371:1–16
Gurer-Orhan H, Sabır HU, Özgüneş H (2004) Correlation between clinical indicators of lead poisoning and oxidative stress parameters in controls and lead-exposed workers. Toxicology 195:147–154
Hasanuzzaman AFM, Robledo D, Gómez-Tato A, Alvarez Dios JA, Harrison PW, Cao A, Fernández-Boo S, Villalba A, Pardo BG, Martínez P (2017) Transcriptomic profile of manila clam (Ruditapes philippinarum) haemocytes in response to Perkinsus olseni infection. Aquaculture 467:170–181
Humphries JE, Yoshino TP (2003) Cellular receptors and signal transduction in molluscan hemocytes: connections with the innate immune system of vertebrates. Integr Comp Biol 43:305–312
Infantino V, Convertini P, Cucci L, Panaro MA, di Noia MA, Calvello R, Palmieri F, Iacobazzi V (2011) The mitochondrial citrate carrier: a new player in inflammation. Biochem J 438:433–436
Jabs T (1999) Reactive oxygen intermediates as mediators of programmed cell death in plants and animals. Biochem Pharmacol 57:231–245
Jha AK, Huang SCC, Sergushichev A, Lampropoulou V, Ivanova Y, Loginicheva E, Chmielewski K, Stewart KM, Ashall J, Everts B, Pearce EJ, Driggers EM, Artyomov MN (2015) Network integration of parallel metabolic and transcriptional data reveals metabolic modules that regulate macrophage polarization. Immunity 42:419–430
Jones DP (2002) [11] redox potential of GSH/GSSG couple: assay and biological significance. Methods Enzymol 348:93–112
Kanehisa M, Goto S (2000) KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res 28:27–30
Kehlet-Delgado H, Richards GP, Häse C, Mueller RS (2017) Three draft genome sequences of vibrio coralliilyticus strains isolated from bivalve hatcheries. Genome Announc 5:e01162–e01117
Kesarcodi-Watson A, Kaspar H, Lategan MJ, Gibson L (2009a) Two pathogens of Greenshell™ mussel larvae, Perna canaliculus: Vibrio splendidus and a V. Coralliilyticus/neptunius-like isolate. J Fish Dis 32:499–507
Kesarcodi-Watson A, Kaspar H, Lategan MJ, Gibson LF (2009b) Challenge of New Zealand Greenshell (TM) mussel Perna canaliculus larvae using two vibrio pathogens: a hatchery study. Dis Aquat Org 86:15–20
Kesarcodi-Watson A, Miner P, Nicolas J-L, Robert R (2012) Protective effect of four potential probiotics against pathogen-challenge of the larvae of three bivalves: Pacific oyster (Crassostrea gigas), flat oyster (Ostrea edulis) and scallop (Pecten maximus). Aquaculture 344–349:29–34
Lambert C, Soudant P, Choquet G, Paillard C (2003) Measurement of Crassostrea gigas hemocyte oxidative metabolism by flow cytometry and the inhibiting capacity of pathogenic vibrios. Fish Shellfish Immunol 15:225–240
Lampropoulou V, Sergushichev A, Bambouskova M, Nair S, Vincent EE, Loginicheva E, Cervantes-Barragan L, Ma X, Huang SCC, Griss T, Weinheimer CJ, Khader S, Randolph GJ, Pearce EJ, Jones RG, Diwan A, Diamond MS, Artyomov MN (2016) Itaconate links inhibition of succinate dehydrogenase with macrophage metabolic remodeling and regulation of inflammation. Cell Metab 24:158–166
Li E, Li C (2014) Use of RNA-seq in aquaculture research. Poult Fish Wildl Sci 2:108–109
Li M, Qiu L, Wang L, Wang W, Xin L, Li Y, Liu Z, Song L (2016) The inhibitory role of γ-aminobutyric acid (GABA) on immunomodulation of Pacific oyster Crassostrea gigas. Fish Shellfish Immunol 52:16–22
Liu X, Ji C, Zhao J, Wu H (2013a) Differential metabolic responses of clam Ruditapes philippinarum to vibrio anguillarum and Vibrio splendidus challenges. Fish Shellfish Immunol 35:2001–2007
Liu X, Zhao J, Wu H, Wang Q (2013b) Metabolomic analysis revealed the differential responses in two pedigrees of clam Ruditapes philippinarum towards Vibrio harveyi challenge. Fish Shellfish Immunol 35:1969–1975
Liu X, Ji C, Zhao J, Wang Q, Li F, Wu H (2014a) Metabolic profiling of the tissue-specific responses in mussel Mytilus galloprovincialis towards Vibrio harveyi challenge. Fish Shellfish Immunol 39:372–377
Liu X, Sun H, Wang Y, Ma M, Zhang Y (2014b) Gender-specific metabolic responses in hepatopancreas of mussel Mytilus galloprovincialis challenged by Vibrio harveyi. Fish Shellfish Immunol 40:407–413
Lu J, Shi Y, Cai S, Feng J (2017) Metabolic responses of Haliotis diversicolor to Vibrio parahaemolyticus infection. Fish Shellfish Immunol 60:265–274
McBean GJ (2012) The transsulfuration pathway: a source of cysteine for glutathione in astrocytes. Amino Acids 42:199–205
Michelucci A, Cordes T, Ghelfi J, Pailot A, Reiling N, Goldmann O, Binz T, Wegner A, Tallam A, Rausell A, Buttini M, Linster CL, Medina E, Balling R, Hiller K (2013) Immune-responsive gene 1 protein links metabolism to immunity by catalyzing itaconic acid production. Proc Natl Acad Sci 110:7820–7825
Milan M, Coppe A, Reinhardt R, Cancela LM, Leite RB, Saavedra C, Ciofi C, Chelazzi G, Patarnello T, Bortoluzzi S, Bargelloni L (2011) Transcriptome sequencing and microarray development for the manila clam, Ruditapes philippinarum: genomic tools for environmental monitoring. BMC Genomics 12:234
Mills E, O’Neill LA (2014) Succinate: a metabolic signal in inflammation. Trends Cell Biol 24:313–320
Mills EL et al (2016) Succinate dehydrogenase supports metabolic repurposing of mitochondria to drive inflammatory macrophages. Cell 167:457–470.e413
Mittal M, Siddiqui MR, Tran K, Reddy SP, Malik AB (2014) Reactive oxygen species in inflammation and tissue injury. Antioxid Redox Signal 20:1126–1167
Moreira R, Balseiro P, Planas JV, Fuste B, Beltran S, Novoa B, Figueras A (2012) Transcriptomics of in vitro immune-stimulated hemocytes from the manila clam Ruditapes philippinarum using high-throughput sequencing. PLoS One 7:e35009
Moreira R, Milan M, Balseiro P, Romero A, Babbucci M, Figueras A, Bargelloni L, Novoa B (2014) Gene expression profile analysis of manila clam (Ruditapes philippinarum) hemocytes after a vibrio alginolyticus challenge using an immune-enriched oligo-microarray. BMC Genomics 15:267
Nikiforova VJ, Willmitzer L (2007) Network visualization and network analysis. In: Baginsky S, Fernie AR (eds) Plant systems biology. Birkhäuser Basel, Basel, pp 245–275
O'Neill LAJ, Kishton RJ, Rathmell J (2016) A guide to immunometabolism for immunologists. Nat Rev Immunol 16:553–565
Ottaviani E, Franchini A, Malagoli D (2010) Inflammatory response in molluscs: cross-taxa and evolutionary considerations. Curr Pharm Des 16:4160–4165
Pauletto M, Milan M, Moreira R, Novoa B, Figueras A, Babbucci M, Patarnello T, Bargelloni L (2014) Deep transcriptome sequencing of Pecten maximus hemocytes: a genomic resource for bivalve immunology. Fish Shellfish Immunol 37:154–165
Philipp EE et al (2012) Massively parallel RNA sequencing identifies a complex immune gene repertoire in the lophotrochozoan Mytilus edulis. PLoS One 7:e33091
Plumb JA, Hanson LA (2011) Health maintenance and principal microbial diseases of cultured fishes. Wiley, New York
Richards GP, Bono JL, Watson MA, Needleman DS (2014) Complete genome sequence for the shellfish pathogen vibrio coralliilyticus RE98 isolated from a shellfish hatchery. Genome Announc 2:e01253–e01214
Richards GP, Watson MA, Needleman DS, Church KM, Häse CC (2015) Mortalities of eastern and Pacific oyster larvae caused by the pathogens vibrio coralliilyticus and vibrio tubiashii. Appl Environ Microbiol 81:292–297
Rosa RD, De Lorgeril J, Tailliez P, Bruno R, Piquemal D, Bachère E (2012) A hemocyte gene expression signature correlated with predictive capacity of oysters to survive vibrio infections. BMC Genomics 13:252
Rowley AF (1996) The evolution of inflammatory mediators. Mediat Inflamm 5:3–13
Rozenblat YB-H, Rosenberg E (2004) Temperature-regulated bleaching and tissue lysis of Pocillopora damicornis by the novel pathogen vibrio coralliilyticus. In: Rosenberg E, Loya Y (eds) Coral health and disease. Springer, Berlin Heidelberg, pp 301–324
Sanders ER (2012) Aseptic laboratory techniques: plating methods. J Vis Exp 63:3064
Schröcksnadel K, Wirleitner B, Winkler C, Fuchs D (2006) Monitoring tryptophan metabolism in chronic immune activation. Clin Chim Acta 364:82–90
Seol HS, Lee SE, Song JS, Lee HY, Park S, Kim I, Singh SR, Chang S, Jang SJ (2016) Glutamate release inhibitor, Riluzole, inhibited proliferation of human hepatocellular carcinoma cells by elevated ROS production. Cancer Lett 382:157–165
Smart KF, Aggio RBM, Van Houtte JR, Villas-Boas SG (2010) Analytical platform for metabolome analysis of microbial cells using methyl chloroformate derivatization followed by gas chromatography-mass spectrometry. Nat Protoc 5:1709–1729
Smith MT, Vermeulen R, Li G, Zhang L, Lan Q, Hubbard AE, Forrest MS, McHale C, Zhao X, Gunn L, Shen M, Rappaport SM, Yin S, Chanock S, Rothman N (2005) Use of ‘Omic’ technologies to study humans exposed to benzene. Chem Biol Interact 153–154:123–127
Song L, Wang L, Qiu L, Zhang H (2010) Bivalve Immunity. In: Söderhäll K (ed) Invertebrate immunity. Springer US, Boston, pp 44–65
Sun Y, Zhang Y, Fu X, Zhang R, Zou J, Wang S, Hu X, Zhang L, Bao Z (2014) Identification of two secreted ferritin subunits involved in immune defense of yesso scallop Patinopecten yessoensis. Fish Shellfish Immunol 37:53–59
Tannahill G et al (2013) Succinate is an inflammatory signal that induces IL-1 [bgr] through HIF-1 [agr]. Nature 496:238–242
Torreilles J, Guérin M-C, Roch P (1996) Reactive oxygen species and defense mechanisms in marine bivalves. C R Acad Sci III 319:209–218
Travers M-A, Boettcher Miller K, Roque A, Friedman CS (2015) Bacterial diseases in marine bivalves. J Invertebr Pathol 131:11–31
Tretter L, Patocs A, Chinopoulos C (2016) Succinate, an intermediate in metabolism, signal transduction, ROS, hypoxia, and tumorigenesis. Biochim Biophys Acta 1857:1086–1101
Venier P, de Pittà C, Bernante F, Varotto L, de Nardi B, Bovo G, Roch P, Novoa B, Figueras A, Pallavicini A, Lanfranchi G (2009) MytiBase: a knowledgebase of mussel (M. Galloprovincialis) transcribed sequences. BMC Genomics 10:72
Villas-Bôas SG, Smart KF, Sivakumaran S, Lane GA (2011) Alkylation or Silylation for analysis of amino and non-amino organic acids by GC-MS? Meta 1:3–20
Voet D, Voet JG, Pratt CW (2016) Fundamentals of biochemistry: life at the molecular level. Wiley, New York
Xia J, Wishart DS (2010) MetPA: a web-based metabolomics tool for pathway analysis and visualization. Bioinformatics 26:2342–2344
Xia J, Sinelnikov IV, Han B, Wishart DS (2015) MetaboAnalyst 3.0—making metabolomics more meaningful. Nucleic Acids Res 43:251–257
Young T, Alfaro AC (2016) Metabolomic strategies for aquaculture research: a primer. Rev Aquac 0:1–31
Young T, Kesarcodi-Watson A, Alfaro AC, Merien F, Nguyen TV, Mae H, le DV, Villas-Bôas S (2017) Differential expression of novel metabolic and immunological biomarkers in oysters challenged with a virulent strain of OsHV-1. Dev Comp Immunol 73:229–245
Yue F, Shi X, Zhou Z, Wang L, Wang M, Yang J, Qiu L, Song L (2013) The expression of immune-related genes during the ontogenesis of scallop Chlamys farreri and their response to bacterial challenge. Fish Shellfish Immunol 34:855–864
Zhang L, Li L, Zhu Y, Zhang G, Guo X (2014) Transcriptome analysis reveals a rich gene set related to innate immunity in the eastern oyster (Crassostrea virginica). Mar Biotechnol 16:17–33
Zhang H, Zhai Y, Yao L, Jiang Y, Li F (2017) Comparative transcriptomics reveals genes involved in metabolic and immune pathways in the digestive gland of scallop Chlamys farreri following cadmium exposure. Chin J Oceanol Limnol 35:603–612
Zhou R, Tardivel A, Thorens B, Choi I, Tschopp J (2010) Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat Immunol 11:136–140
Zitka O et al (2012) Redox status expressed as GSH: GSSG ratio as a marker for oxidative stress in paediatric tumour patients. Oncol Lett 4:1247–1253
Acknowledgements
We would like to thank Westpac Mussel Distributors Ltd. for supplying mussels, Aditya Kesarcodi-Watson (Cawthron Institute, Nelson, New Zealand) for providing the bacterial strain and Erica Zarate and Saras Green (University of Auckland) for their assistance with metabolite sample processing. This project was supported by the New Zealand Ministry of Business, Innovation and Employment (MBIE) (CAWX1315). Additional financial support was provided by a New Zealand ASEAN Scholarship and an award from the New Zealand Marine Sciences Society (NZMSS) to T. V. Nguyen, under the supervision of A. C. Alfaro and F. Merien. We are also thankful to all the members of the Aquaculture Biotechnology Research Group at the Auckland University of Technology (ABRG-AUT) for their assistant during this project.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Table S1
(DOCX 19 kb)
Rights and permissions
About this article
Cite this article
Nguyen, T.V., Alfaro, A.C., Young, T. et al. Metabolomics Study of Immune Responses of New Zealand Greenshell™ Mussels (Perna canaliculus) Infected with Pathogenic Vibrio sp.. Mar Biotechnol 20, 396–409 (2018). https://doi.org/10.1007/s10126-018-9804-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10126-018-9804-x