Abstract
Background
For many cancer resections, a hospital volume-outcome relationship exists. The data regarding gastric cancer resection—especially in the western hemisphere—are ambiguous. This study analyzes the impact of gastric cancer surgery caseload per hospital on postoperative mortality and failure to rescue in Germany.
Methods
All patients diagnosed with gastric cancer from 2009 to 2017 who underwent gastric resection were identified from nation-wide administrative data. Hospitals were grouped into five equal caseload quintiles (I–V in ascending caseload order). Postoperative deaths and failure to rescue were determined.
Results
Forty-six thousand one hundred eighty-seven patients were identified. There was a significant shift from partial resections in low-volume hospitals to more extended resections in high-volume centers. The overall in-house mortality rate was 6.2%. The crude in-hospital mortality rate ranged from 7.9% in quintile I to 4.4% in quintile V, with a significant trend between volume categories (p < 0.001). In the multivariable logistic regression analysis, quintile V hospitals (average of 29 interventions/year) had a risk-adjusted odds ratio of 0.50 (95% CI 0.39–0.65), compared to the baseline in-house mortality rate in quintile I (on average 1.5 interventions/year) (p < 0.001). In an analysis only evaluating hospitals with more than 30 resections per year mortality dropped below 4%. The overall postoperative complication rate was comparable between different volume quintiles, but failure to rescue (FtR) decreased significantly with increasing caseload.
Conclusion
Patients who had gastric cancer surgery in hospitals with higher volume had better outcomes and a reduced failure to rescue rates for severe complications.
Similar content being viewed by others
Introduction
The incidence, mortality and treatment of gastric cancers vary considerably around the world [1, 2], with surgical resection being the therapeutic cornerstone for patients with non-metastatic disease [3]. Unlike for pancreatic, esophageal, pulmonary and colorectal resections, however, the correlation between individual surgeon and hospital volume with both short-term and long-term outcomes for gastric resection is unclear [4,5,6,7,8], with the notable exception of East Asia where the incidence of gastric cancer is high [9]. Indeed, a recent review of gastric cancer trials and studies on gastric cancer resections performed mainly in Western countries on more than one thousand patients failed to establish a correlation between hospital volume and patient outcomes [10]. Additionally, real-world data does not suggest reduced mortality or improved long-term survival for gastric cancer resections in high-volume centers [11,12,13]. According to an analysis of gastric resections in Germany from between 2010 and 2015, overall in-house mortality was high (around 10%), decreasing slightly when annual surgical volume increased [14,15,16]. However, this analysis did not distinguish between the different indications for gastric resection.
The aim of this study was therefore to investigate the association between hospital volume for gastric cancer surgery and postoperative outcome by dividing hospitals into five caseload quintile groups. The primary endpoint was in-house mortality while secondary endpoints were rate of complications and associated failure to rescue.
Methods
Case definition and hospital volume
This is a retrospective cohort study based on the individual inpatient billing data from the nation-wide German diagnosis-related groups (DRG) registry. All patients with a DRG code C16 for gastric cancer as the main diagnosis and a concomitant procedure code indicating major gastric or gastric and partial esophageal resection (OPS codes 5435/6/7/8, 542411/12, 54241/2) between 1st January 2009 and 31st December 2017 were included. Procedures were considered hierarchically within each patient and the more radical intervention was considered as the principal intervention to avoid double counting of interventions. ICD-codes for complications were identified among patients’ secondary diagnoses. A dichotomous variable for complications was created which became positive if one or more complications were recorded for an inpatient case. Hospitals were divided into five volume quintile categories of approximately equal size based on their pooled number of gastric resections in this period as previously described (quintiles I–V in ascending caseload order) [6, 7]. As a sensitivity analysis hospital volume was analyzed as a continuous variable and based on annual caseload (≤ 10, 11–29, ≥ 30 cases/year). Primary endpoint was defined as post-operative death within the same in-house stay as gastric cancer resection. Secondary endpoints were occurrence of complications and death after occurrence of defined complications (FtR).
Data
Anonymized data was accessed via the Research Data Centre of the Federal Statistical Office by controlled remote data analysis. Data included in the analysis consisted of anonymized unique patient and hospital identifiers, primary and secondary DRG codes, concomitant procedure codes, patient sex, age, duration of mechanical ventilation, mass transfusion and length of stay (LOS). The German adaptation of the ICD-10-GM codes as well as the German procedure codes in the relevant versions were used for this study [17]. If duplicate patient identifiers occurred, one data set was randomly chosen and included in the analysis. Only elective procedures were considered.
Potential confounding
For each patient a comorbidity score, as published by Stausberg and Hagn [18] was determined. Patient data was cross tabulated and crude associations were evaluated using χ2 tests where appropriate. Trends across hospital volume quintiles and temporal trends were assessed with a non-parametric test for trend [19]. Crude odds ratios (OR) between in-hospital mortality and the main independent variable (hospital volume quintile), as well as with other secondary variables were computed to identify potential confounders. Mantel–Haenszel method was used to screen for relevant effect modification. To detect multicollinearity, we determined the correlation between the different pairs of variables.
Multivariable regression
We estimated the effect of hospital volume on in-hospital mortality by multivariable logistic regression analysis considering patient clustering within institutions by means of hospital as a random effect. The accuracy of the random-effects estimators of the multivariable models was checked by refitting the models for different numbers of quadrature points and subsequent comparison of the values of the estimators. 10–4 was considered to be the maximum acceptable relative difference between the different quadrature points. The multivariable model was adjusted for known confounding effects of sex, age and co-morbidity. We also fitted models with patient number per hospital as a continuous variable. Model performance was assessed using likelihood ratio tests. Risk standardized mortality rates (RSMR), risk standardized major complication rates (RSMCR) and a combined risk standardized mortality after major complication rate (RSMMCR) for each hospital based on predicted mortality rates were calculated by multivariable logistic regression as described in [20]. Differences in means were compared by Kruksal–Wallis tests.
Statistical analysis
The statistical evaluation was carried out with Stata 14.2 (StataCorp LP, College Station, Texas, USA). A p value ≤ 0.05 was considered significant. The authors followed RECORD Guidelines for good practice of secondary data analysis [21].
Results
A total of 46,218 datasets with the diagnosis of gastric cancer (ICD code C16) and gastric resection (procedure codes 5435/6/7/8, 542411/12, 54241/2) in Germany between 1 January 2009 and 31 December 2017 were identified. 31 cases were excluded from analysis due to duplication (0.07%) leaving 46,187 patients for further analysis.
Patient background
Seventeen thousand eight hundred sixty-four patients (38.7%) were female, and the median age was 70 years. 27.9% of patients (n = 12,881) had cancer of the pyloric antrum, followed by cancer of the corpus (n = 11,789, 25.5%) and the cardia (n = 10,046, 21.8%). Most frequently, patients underwent total gastrectomy (n = 27,717; 60.0%), followed by subtotal or 4/5 gastric resection (n = 10,080; 21.8%) and partial, or 2/3 gastric resection (n = 3370; 7.9%) (Table 1). A significant temporal trend could not be observed regarding patient age. The mean comorbidity score steadily increased during the observation period (102.07 in 2009 versus 103.19 in 2017, p < 0.001). The median LOS steadily decreased (19 days in 2009 versus 16 days in 2017, p < 0.001).
Unadjusted in-hospital mortality
Nationwide in-hospital mortality following elective gastric cancer resection was 6.2% (n = 2872 of 46,187) (Table 2). The mortality rate was higher for men than for women (6.7% (1908 of 28,323) versus 5.4% (964 of 17,864), respectively) and increased with patient age. While patients under 55 years of age had a mortality rate of 1.5% (100 of 6626), this rate increased to 4.6% (1069 of 23,246) for patients between 55 and 74 years of age and increased further to 10.4% in patients over 75 years of age (1703 of 16,315 or 10.4%). When considering the type of resection, the highest in-hospital mortality was observed in patients receiving a partial gastric resection (265 of 3370; 7.9%), followed by gastrectomy with concomitant esophageal resection (162 of 2134; 7.6%). The in-house mortality for patients after total gastrectomy and subtotal gastric resection was around 6.0% (1650 of 27,717 and 126 of 1913 respective).
The 1084 hospitals were divided into 5 volume quintile groups of equal size (mean 9237 patients per quintile, maximum absolute difference of 5.4% between volume quintiles). 665 hospitals were in quintile I, whereas 73 and 36 hospitals were in quintiles IV and V (Table 2, Fig. 1a), respectively. Median LOS was 20 days in quintile I compared to 16 days in quintile V hospitals. Median patient age steadily decreased from 73 to 67 years with increasing hospital volume (very-low versus very-high-volume centers).
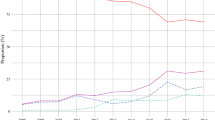
Left row: a Hospital caseload per volume category. b Unadjusted in-house mortality and 95% CI; c adjusted in-house mortality and 95% CI according to hospital volume. Right row Number of gastric resections (gray bar) and in-house mortality (black line) according to hospital quintile. d subtotal gastrectomy; e gastrectomy; f extended resections
Hospital volume quintiles
Annually, a mean of 1.5 patients underwent gastric cancer resection in quintile I hospitals. Quintile V hospitals performed 28.8 resections per year. Postoperative mortality during hospital stay was inversely associated with hospital volume. The crude in-house mortality rate ranged from 7.9% (716 of 9100) in hospitals in the lowest volume category to 4.4% (414 of 9336) in quintile V centers (p < 0.001 for trend) (Table 2).
Accounting for patient clustering within institutions and for the effect of confounding, a steady and significant decrease in in-hospital mortality was observed with increasing volume of gastric cancer resections. The adjusted OR for postoperative in-hospital mortality was almost 50% lower in quintile V, 34% lower in quintile IV, and 24% lower in quintile III hospitals as compared to quintile I hospitals (p < 0.001 for Quintile IV and V; p = 0.004 for Quintile III) (Fig. 1b–f; Table 3).
Postoperative complications and failure to rescue
Mechanical ventilation for ≥ 48 h, massive transfusion of ≥ 6 units of packed red blood cells and relaparotomy were all associated with a significant increase in in-hospital death [mechanical ventilation 39.0% versus 3.1% (1565 of 4015 and 1307 of 42,172), massive transfusion 31.4% versus 3.8% (1271 of 4042 and 1601 of 42,145) and relaparotomy 28.0% versus 4.5% (942 of 3369 and 1930 of 42,818), all p < 0.001] (Table 4). Anastomotic leakage was reported in 4.2% of patients and of those 27.5% died during their inpatient time (536 of 1947 as compared to 5.3% mortality (2336 of 41,904) in patients without anastomotic leakage; p < 0.001). Peritonitis occurred in 10.4% of cases (4817) and was also significantly associated with increased mortality (1491 of 4817; 31.0%, p < 0.001). There were several cases of mediastinitis (137 of 46,187) and pleural empyema (485 of 46,187), which were also associated with an increased fatal outcome (mediastinitis: 54 of 137; 39.4%; pleural empyema: 120 of 485; 24.7%; both p < 0.001). Pancreatitis was reported in 816 patients (Mortality: n = 214, 26.2%; p < 0.001 for increase in mortality). 5.7% (n = 2623) of all patients were diagnosed with surgical site infection (SSI) (Mortality: n = 372; 14.2%, p < 0.001 for increase in mortality). Mortality in patients with clostridia difficile infection was doubled compared to patients without (86 of 623 versus 2786 of 456,564 or 13.8 versus 6.1%, p < 0,001) (supp. Table 1).
Anastomotic leakage was reported significantly more often in quintile III and V hospitals, with a rate of 4.9% in quintile V as compared to a rate of 3.5% in quintile I (p < 0.001 for differences across categories). Prolonged ventilation occurred with similar frequency in all volume categories between around 8.5 and 9.0% across all volume categories. Relaparotomy was most frequent in quintile IV and V centers (8.1% and 7.7% versus 6.6% in quintile I, p = 0.003). Pulmonary embolism occurred more often in quintile IV as compared to quintile I (2.4% versus 1.3%, p < 0.001). Peritonitis, stroke, myocardial infarction, the incidence of bleeding and transfusion, cardio pulmonal resuscitation or acute kidney injury were not significantly associated with hospital volume. There was no significant difference in the occurrence of SSI which was reported in 5.4–5.9% of all patients.
Although anastomotic leak and relaparotomy were more common in higher-volume hospitals, mortality rates in patients with anastomotic leak and relaparotomy decreased with increasing hospital volume, both ranging from around 30% in low-volume hospitals to around 20% in the highest-volume hospitals (p < 0.002) (Table 4). Similarly, pulmonary embolism was significantly more frequent in quintile V; however, patients treated in these hospitals displayed a significantly lower in-hospital mortality rate (15.4% versus 29.7%, p = 0.032) (Table 4). In patients with prolonged ventilation, the failure to rescue (FtR) was equally significantly lower in a high-volume center (32.9% versus 42.6%, p = 0.001) (Fig. 2, supp. Table 1; Table 4). Mortality in patients with peritonitis and SSI was also lower in centers with a higher caseload (peritonitis: 26.4% versus 32.9%; SSI: 9.8% versus 17.8%, p = 0.008 and 0.002 respectively).
Because patient characteristics as well as operative characteristics differ significantly between hospitals, we calculated the RSMCR which ranged between 6.1 and 6.2%. Both RSMR and RSMMCR significantly decreased from 7.5 to 4.4% and 7.4–4.6%, respectively, as hospital caseload increased from the lowest to the highest-volume category (both p < 0.001) (Supp. Table 2).
Sensitivity analysis—caseload ≥ 30 cases/year
Overall annual numbers of gastric cancer resection per hospital in Germany are far below the average in Asia. As more than 30 resections per year are considered as “high volume” in Europa and as we observed a non-linear drop in quintile V (29 resections per year), we performed a sensitivity analysis dividing hospitals in 3 caseload groups (≤ 10, 11–29, ≥ 30 cases/year) [22]. Most patients underwent gastric cancer resection in hospitals performing 10 or less resections annually (n = 25,538 patients, 55.3%, 953 hospitals). Fourteen hospitals performed an annual average of ≥ 30 resections and treated 10.7% of all patients (n = 4919). Crude in-house mortality dropped to 3.9% in these hospitals (as compared to a crude mortality of 6.9% and 5.8% in hospitals with ≤ 10 and 11–29 annual resections respectively, p < 0.001 for trend). Multivariable regression analysis displayed a 25% and 49.9% lower adjusted OR for in-house-mortality (Table 5).
When analyzing postoperative complications as well as the FtR in three caseload groups, comparable results of a significantly reduced mortality for nearly all complications in case of occurrence were observed (Table 5).
Discussion
This nation-wide analysis establishes a significant and strong correlation between hospital volume and in-hospital mortality for patients with gastric cancer surgery in Germany. In hospitals with the highest caseload (approximately 29 surgeries performed annually for gastric carcinoma), the adjusted OR for in-hospital mortality was 0.50 (95% CI 0.39–0.65) compared to the lowest volume quintile I hospitals that perform less than two surgeries for gastric carcinoma each year. This difference in mortality was found in both the unadjusted and the adjusted analysis for known confounders such as age, sex and comorbidities. Furthermore, mortality displayed a nearly linear correlation with the annual caseload for each hospital even though the proportion of extended resection increased with the annual caseload. Compared to the situation in East Asia, hospital volume in Germany—even in volume-quintiles IV and V, representing the centers with the highest caseload—was relatively small. We, therefore, conducted a sensitivity analysis focusing on hospitals with an annual average of ≥ 30 resections/year. Overall, only 14 hospitals are in this group confirming our strategy of dividing the population in five quintiles. The in-house mortality in these hospitals drops below 4%.
Whereas overall mortality is higher in extended resection compared to gastrectomy or 4/5 gastrectomy, a relatively small group of patients undergoing 2/3 gastrectomy display an even higher mortality. This could potentially be explained by the fact that 2/3 is not a standard resection for gastric cancer and is only applied in patients with elevated risk, or if the gastric cancer diagnosis was by coincidence after resection due to other indications.
Though the postoperative complication rate partly increased with hospital volume, probably due to more complex surgical procedures in higher-volume hospitals, the failure to rescue after both surgical (anastomotic leakage and peritonitis) and non-surgical (such as pulmonary embolism) complications decreased. Interestingly, for several complications there were only minor differences in the FtR in quintiles I–III and a sharp drop for quintile IV and V hospitals. This non-linear correlation was even more obvious when analyzing hospitals with ≤ 10, 11–29, ≥ 30 cases per year. This non-linear reduced FtR argues that a certain threshold is important to reduce preoperative mortality. Lower FtR rates could also reflect structural differences between high- and low-volume hospitals, for instance availability of specialized intensive care units or availability of computed tomography.
So far, the data regarding the impact of the annual case load of gastric cancer surgery on the in-house mortality are conflicting [23]. Whereas some studies demonstrated a volume-outcome correlation, there are numerous reports which could not demonstrate differences especially when analyzing the volume impact on gastric and esophageal cancer resection [11, 13, 24]. Interestingly, studies demonstrating a volume-outcome relationship have two major distinct characteristics. First, they analyzed large databases with several thousand patients comparable to our study [23, 25, 26]. Second, the relative difference in annual caseload between the quintiles is high. In studies that found no difference, the highest quintile comprises roughly more than 20 patients per year, whereas in studies demonstrating differences, high-volume hospitals treated more than 80 patients per year [11, 24, 26]. A possible explanation for this is that studies not finding differences so far analyzed data from hospitals under a certain annual case threshold after which the surgical mortality might have dropped. This would be in line with our study showing that the difference between the quintiles is mainly based on a reduced FtR rather than a lower complication rate, and that the failure to rescue shows a non-linear correlation. A study from the Netherlands demonstrated that the main impact on gastric cancer surgery survival is the teaching status of the hospital (university hospitals perform better than non-teaching hospitals), rather than the annual case load [27].
Our study had some strengths. First, we used a complete national sampling cohort independent of insurance status to investigate the association between hospital volume for gastric cancer and postoperative mortality. Thus, the results of this study are unbiased and provide evidence-based arguments for optimal management of gastric cancer patients requiring surgical treatment. Second, the analyzed data consisted of the billing data, which is usually crosschecked by the medical service of health insurance companies as an external audit thereby serving to increase data quality. Third, the endpoint of in-house mortality does not have a documentation bias. Nevertheless, it has been demonstrated for several cancer surgeries, that in-house mortality could be inferior to 30- or 90-day mortality due to the fact that death occurring after discharge could be missed [28].
Our analysis was not limited by selection biases in analyzing a large and representative population-based register. Since the study is based on observational data, only associations, rather than causal relationships, can be reported. Long-term outcomes such as follow-up, survival or important clinical markers such as tumor stage are not recorded in the registry data. Other important demographics were not captured in this dataset and could not be accounted for. Another limitation is that data quality of population-based registries, especially for occurrence of complication, is inferior compared to clinical registries or data from clinical studies due to missing of grading of complications as well as management and outcome data.
Comparing the survival of gastric cancer patients between East Asia and the Western hemisphere shows a worse outcome both in short-term and long-term survival in the latter, which in part may be due to different tumor stage as well as differences in biological behavior [10, 14]. Nevertheless, this difference in survival could be decreased by increasing the annual case load in hospitals and encouraging centralization. Based on our data, the threshold of 10 surgical cases in Germany and 20 in The Netherlands should be further evaluated in terms of significant improvement of perioperative outcome.
References
Collaborators GBDSC. The global, regional, and national burden of stomach cancer in 195 countries, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol. 2020;5(1):42–54. https://doi.org/10.1016/S2468-1253(19)30328-0 (Epub 2019/10/28; PubMed PMID: 31648970; PubMed Central PMCID: PMCPMC7033564).
Yamamoto M, Rashid OM, Wong J. Surgical management of gastric cancer: the East vs. West perspective. J Gastrointest Oncol. 2015;6(1):79–88. https://doi.org/10.3978/j.issn.2078-6891.2014.097 (Epub 2015/02/03; PubMed PMID: 25642341; PubMed Central PMCID: PMCPMC4294827).
Ajani JA, D’Amico TA, Almhanna K, Bentrem DJ, Chao J, Das P, et al. Gastric cancer, Version 3.2016, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2016;14(10):1286–312. https://doi.org/10.6004/jnccn.2016.0137 (Epub 2016/10/05; PubMed PMID: 27697982).
Ghaferi AA, Birkmeyer JD, Dimick JB. Hospital volume and failure to rescue with high-risk surgery. Med Care. 2011;49(12):1076–81. https://doi.org/10.1097/MLR.0b013e3182329b97 (Epub 2011/10/18; PubMed PMID: 22002649).
Birkmeyer JD, Siewers AE, Finlayson EV, Stukel TA, Lucas FL, Batista I, et al. Hospital volume and surgical mortality in the United States. N Engl J Med. 2002;346(15):1128–37. https://doi.org/10.1056/NEJMsa012337 (Epub 2002/04/12; PubMed PMID: 11948273).
Diers J, Wagner J, Baum P, Lichthardt S, Kastner C, Matthes N, et al. Nationwide in-hospital mortality rate following rectal resection for rectal cancer according to annual hospital volume in Germany. BJS Open. 2020. https://doi.org/10.1002/bjs5.50254.
Diers J, Wagner J, Baum P, Lichthardt S, Kastner C, Matthes N, Löb S, Matthes H, Germer C-T, Wiegering A. Nationwide in-hospital mortality following colonic cancer resection according to hospital volume in Germany. 2019. PMID: 31592096 PMCID: PMC6773649. https://doi.org/10.1002/bjs5.50173
Baum P, Diers J, Haag J, Klotz L, Eichhorn F, Eichhorn M, et al. Nationwide effect of high procedure volume in lung cancer surgery on in-house mortality in Germany. Lung Cancer. 2020. https://doi.org/10.1016/j.lungcan.2020.08.018.
Choi H, Yang SY, Cho HS, Kim W, Park EC, Han KT. Mortality differences by surgical volume among patients with stomach cancer: a threshold for a favorable volume-outcome relationship. World J Surg Oncol. 2017;15(1):134. https://doi.org/10.1186/s12957-017-1203-7 (Epub 2017/07/19; PubMed PMID: 28716145; PubMed Central PMCID: PMCPMC5513131).
Mukai Y, Kurokawa Y, Takiguchi S, Mori M, Doki Y. Are treatment outcomes in gastric cancer associated with either hospital volume or surgeon volume? Ann Gastroenterol Surg. 2017;1(3):186–92. https://doi.org/10.1002/ags3.12031 (Epub 2018/06/05. PubMed PMID: 29863147; PubMed Central PMCID: PMCPMC5881359).
Dikken JL, Dassen AE, Lemmens VE, Putter H, Krijnen P, van der Geest L, et al. Effect of hospital volume on postoperative mortality and survival after oesophageal and gastric cancer surgery in The Netherlands between 1989 and 2009. Eur J Cancer. 2012;48(7):1004–13. https://doi.org/10.1016/j.ejca.2012.02.064 (Epub 2012/03/30. PubMed PMID: 22456179).
Song Y, Tieniber AD, Roses RE, Fraker DL, Kelz RR, Karakousis GC. National trends in centralization and perioperative outcomes of complex operations for cancer. Surgery. 2019;166(5):800–11. https://doi.org/10.1016/j.surg.2019.03.025 (Epub 2019/06/25. PubMed PMID: 31230839).
Anderson O, Ni Z, Moller H, Coupland VH, Davies EA, Allum WH, et al. Hospital volume and survival in oesophagectomy and gastrectomy for cancer. Eur J Cancer. 2011;47(16):2408–14. https://doi.org/10.1016/j.ejca.2011.07.001 (Epub 2011/08/13. PubMed PMID: 21835609).
Baum P, Diers J, Lichthardt S, Kastner C, Schlegel N, Germer CT, et al. Mortality and Complications Following Visceral Surgery. Dtsch Arztebl Int. 2019;116(44):739–46. https://doi.org/10.3238/arztebl.2019.0739 (Epub 2019/11/28. PubMed PMID: 31774053; PubMed Central PMCID: PMCPMC6912125).
Nimptsch U, Haist T, Gockel I, Mansky T, Lorenz D. Complex gastric surgery in Germany-is centralization beneficial? Observational study using national hospital discharge data. Langenbecks Arch Surg. 2019;404(1):93–101. https://doi.org/10.1007/s00423-018-1742-6 (Epub 2018/12/16. PubMed PMID: 30552508).
Hendricks A, Diers J, Baum P, Weibel S, Kastner C, Müller S, Lock JF, Köhler F, Meybohm P, Kranke P, Germer CT, Wiegering A. Systematic review and meta-analysis on volume-outcome relationship of abdominal surgical procedures in Germany. Int J Surg. 2021;9(86):24–31. https://doi.org/10.1016/j.ijsu.2020.12.010. Online ahead of print. PMID: 33429078 Review.
Information DIfMDu. Available from: https://www.dimdi.de/static/de/klassi/icd-10-gm/kodesuche/vorgaenger.htm.
Stausberg J, Hagn S. New morbidity and comorbidity scores based on the structure of the ICD-10. PLoS ONE. 2015;10(12):e0143365. https://doi.org/10.1371/journal.pone.0143365 (Epub 2015/12/15. PubMed PMID: 26656501; PubMed Central PMCID: PMCPMC4677989).
Cuzick J. A Wilcoxon-type test for trend. Stat Med. 1985;4(1):87–90. https://doi.org/10.1002/sim.4780040112 (Epub 1985/01/01. PubMed PMID: 3992076).
Lenzi J, Pildava S. Tips for calculating and displaying risk-standardized hospital outcomes in Stata. Stata J. 2019;19(2):477–96. https://doi.org/10.1177/1536867x19854021 (PubMedPMID: WOS:000486589100008).
Benchimol EI, Smeeth L, Guttmann A, Harron K, Moher D, Petersen I, et al. The REporting of studies Conducted using Observational Routinely-collected health Data (RECORD) statement. PLoS Med. 2015;12(10):e1001885. https://doi.org/10.1371/journal.pmed.1001885 (Epub 2015/10/07; PubMed PMID: 26440803; PubMed Central PMCID: PMCPMC4595218).
Claassen YHM, van Sandick JW, Hartgrink HH, Dikken JL, De Steur WO, van Grieken NCT, et al. Association between hospital volume and quality of gastric cancer surgery in the CRITICS trial. Br J Surg. 2018;105(6):728–35. https://doi.org/10.1002/bjs.10773 (Epub 2018/04/14. PubMed PMID: 29652082).
Dikken JL, Stiekema J, van de Velde CJ, Verheij M, Cats A, Wouters MW, et al. Quality of care indicators for the surgical treatment of gastric cancer: a systematic review. Ann Surg Oncol. 2013;20(2):381–98. https://doi.org/10.1245/s10434-012-2574-1 (Epub 2012/10/12. PubMed PMID: 23054104).
Coupland VH, Lagergren J, Luchtenborg M, Jack RH, Allum W, Holmberg L, et al. Hospital volume, proportion resected and mortality from oesophageal and gastric cancer: a population-based study in England, 2004–2008. Gut. 2013;62(7):961–6. https://doi.org/10.1136/gutjnl-2012-303008 (Epub 2012/10/23. PubMed PMID: 23086798).
Gabriel E, Narayanan S, Attwood K, Hochwald S, Kukar M, Nurkin S. Disparities in major surgery for esophagogastric cancer among hospitals by case volume. J Gastrointest Oncol. 2018;9(3):503–16. https://doi.org/10.21037/jgo.2018.01.18 (Epub 2018/07/13. PubMed PMID: 29998016; PubMed Central PMCID: PMCPMC6006045).
Nomura E, Tsukuma H, Ajiki W, Oshima A. Population-based study of relationship between hospital surgical volume and 5-year survival of stomach cancer patients in Osaka. Japan Cancer Sci. 2003;94(11):998–1002. https://doi.org/10.1111/j.1349-7006.2003.tb01391.x (Epub 2003/11/13. PubMed PMID: 14611678).
Dikken JL, Wouters MW, Lemmens VE, Putter H, van der Geest LG, Verheij M, et al. Influence of hospital type on outcomes after oesophageal and gastric cancer surgery. Br J Surg. 2012;99(7):954–63. https://doi.org/10.1002/bjs.8787 (Epub 2012/05/10. PubMed PMID: 22569956).
Damhuis RA, Wijnhoven BP, Plaisier PW, Kirkels WJ, Kranse R, van Lanschot JJ. Comparison of 30-day, 90-day and in-hospital postoperative mortality for eight different cancer types. Br J Surg. 2012;99(8):1149–54. https://doi.org/10.1002/bjs.8813 (Epub 2012/06/22. PubMed PMID: 22718521).
Acknowledgements
The authors thank Dr. Mohammed Hankir for language editing.
Funding
No funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Diers, J., Baum, P., Wagner, J.C. et al. Hospital volume following major surgery for gastric cancer determines in-hospital mortality rate and failure to rescue: a nation-wide study based on German billing data (2009–2017). Gastric Cancer 24, 959–969 (2021). https://doi.org/10.1007/s10120-021-01167-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10120-021-01167-8