Abstract
Background
Microsatellite instability (MSI) and programmed death-ligand 1 (PD-L1) are candidate predictors for the response to immune checkpoint inhibitors, and may predict chemotherapy sensitivity. We investigated the simultaneous expression of mutL homolog 1 (MLH1), a mismatch repair gene, and PD-L1 in gastric cancers.
Methods
We examined MLH1 and PD-L1 expression in surgical specimens from 285 gastric cancer patients treated with or without preoperative chemotherapy, and assessed the relation between expression results and both histological response and recurrence-free survival (RFS).
Results
Of 285 patients, 28 (9.8%) and 70 (24.6%) exhibited negative MLH1 and high PD-L1 expression, respectively. Most MLH1-negative tumors (85.7%) showed high MSI, and these tumors exhibited high PD-L1 expression more frequently than MLH1-positive tumors (57.1% vs. 21.0%, P < 0.001). MLH1-negative patients were significantly less likely to respond to preoperative chemotherapy than MLH1-positive patients (16.7% vs. 61.2%, P = 0.005), whereas there was no significant difference between high- and low-PD-L1 expression patients (55.9% vs. 56.6%, P = 0.95). RFS in patients without preoperative chemotherapy was significantly longer in the MLH1-negative group than in the MLH1-positive group (HR 0.30; 95% CI 0.09–0.95; P = 0.030), whereas in patients with preoperative chemotherapy there was no significant difference in RFS between the two groups (HR 0.70; 95% CI 0.30–1.63; P = 0.41). PD-L1 expression was not associated with RFS in patients with or without chemotherapy.
Conclusions
Loss of MLH1 was associated with chemoresistance and did not prolong survival following neoadjuvant chemotherapy. The strong association between MLH1 and MSI status suggests that immune checkpoint inhibitors may be preferable to conventional chemotherapy for MLH1-negative gastric cancer.
Similar content being viewed by others
Introduction
Gastric cancer is still one of the major causes of cancer-related deaths worldwide [1]. While curative surgical resection has long been a standard treatment for gastric cancer [2], the recurrence rate is still high; therefore, preoperative and/or postoperative adjuvant chemotherapy is routinely performed for advanced yet resectable cases [3, 4]. However, few predictive biomarkers for the response to chemotherapy in gastric cancer patients have been identified.
Recently, the Cancer Genome Atlas Research Network performed a comprehensive molecular characterization of gastric cancer and proposed microsatellite instability (MSI) as a genomic subtype [5]. In colorectal cancer, high microsatellite instability, or MSI-H, is a specific feature of hereditary nonpolyposis colorectal cancer (HNPCC), which is caused by mismatch repair deficiency (MMRD) resulting from a germline mutation in one of the MMR genes, including mutL homolog 1 (MLH1) [6]. Meanwhile, it is known that the hypermethylation in the promoter regions of the MLH1 gene, caused mainly by Helicobacter pylori infection, is strongly associated with MMRD in gastric cancer, leading to MSI-H status [7].
MSI-H status has been reported to be associated with a good prognosis and poor response to fluorouracil-based adjuvant chemotherapy in colorectal cancer [8, 9]. In gastric cancer, correlative studies of two randomized controlled trials of adjuvant chemotherapy for resectable gastric cancer suggested that MSI-H status might be a negative predictor of prognostic benefit from adjuvant chemotherapy [10, 11]. However, a direct correlation between MSI-H and chemosensitivity, particularly histological response, has not yet been confirmed. In this study, we examined the expression of MLH1, the inactivation of which is a main cause of MMRD, to determine if it predicted MSI status and if it was associated with prognosis and histological response to preoperative chemotherapy in resectable gastric cancer.
In addition, MSI-H was previously suggested to be associated with the expression of programmed death-ligand 1 (PD-L1), a molecule that has attracted much attention as a predictive factor for the response to immune checkpoint inhibitors [12, 13]. Therefore, we also investigated the association between PD-L1 expression and both prognosis and preoperative chemotherapy response in gastric cancer.
Methods
Patients
We collected the data of 110 consecutive patients treated with preoperative chemotherapy between January 2008 and December 2016, and 175 consecutive patients treated without preoperative chemotherapy between January 2008 and December 2012. Patients who underwent non-curative resection (R2) were excluded. All tumors were histologically diagnosed as adenocarcinoma of the stomach. A total of 285 gastric cancer tissue samples were used after written informed consent was obtained from each patient. We used the 14th edition of the Japanese classification of gastric carcinoma to determine the pathological stage [14]. Since 2008, our institution has administered two or three cycles of preoperative, fluorouracil-based doublet or triplet chemotherapy regimens in patients with cStage III or IV. This study was approved by the Institutional Review Board of Osaka University Hospital.
Evaluation of histological response
The histological response to preoperative chemotherapy was evaluated based on the proportion of viable cancer cells according to the Japanese Gastric Cancer Association criteria, and categorized into five grades: grade 3, no viable tumor cells remain; grade 2, viable tumor cells remain in less than 1/3 of the tumor area; grade 1b, viable tumor cells remain in more than 1/3 but less than 2/3 of the tumor area; grade 1a, viable tumor cells occupy more than 2/3 of the tumor area; grade 0, no evidence of treatment effect [14]. Patients with histological response grade 3 were excluded from the study, because we could not evaluate MLH1 or PD-L1 expression in resected specimens. Patients with histological grade 2 or 1b were defined as responders, and those with grade 1a or 0 as non-responders, in accordance with a previous study [15].
Evaluation of MLH1 expression
For MLH1 immunohistochemistry, 3.5-µm-thick sections were prepared from formalin-fixed, paraffin-embedded (FFPE) blocks. The tissue slides were deparaffinized in xylene and then rehydrated through graded ethanol solutions. Antigen was retrieved in 10 mM citrate buffer (pH 6.0) at 110 °C for 15 min. Endogenous peroxidase activity was blocked by incubation in 0.3% hydrogen peroxide for 20 min at room temperature. The slides were then incubated at 4 °C overnight with monoclonal antibody against MLH1 (a mouse anti-MLH1 monoclonal antibody, cat. no. 550838; clone G168-15; dilution, 1:100; BD Biosciences, Franklin Lakes, NJ, USA). Color was developed by incubating the sections in 3,3′-diaminobenzidine tetrahydrochloride (DAB) with 0.05% hydrogen peroxide for 3 min. Finally, the slides were counterstained with 0.1% hematoxylin for 30 s. Normal human tonsil tissue was used as a positive control.
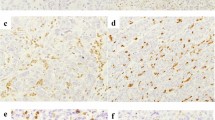
For determining MLH1 expression, normal expression was defined as the presence of nuclear staining of tumor cells irrespective of the proportion or intensity, and the nuclear staining of each cancer cell was evaluated in comparison to that of normal epithelium and the positive control. As reported previously [10, 16], negative MLH1 expression was designated when nuclear staining was not present in any tumor cells, but was observed in normal epithelium and the positive control (Fig. 1a, b). Immunohistochemistry examination was independently performed by two of the authors (T.H. and J.I.), who were blinded to the clinical data.
Representative immunohistochemical staining with MLH1 and PD-L1 in gastric cancer. Nuclear-stained cancer cells negative for MLH1 (a). Nuclear-stained cancer cells positive for MLH1 (b). Membrane-stained cancer cells negative for PD-L1 (c). Membrane-stained cancer cells positive for PD-L1 (d). All images were taken at an original magnification of × 200
Evaluation of PD-L1 expression
For PD-L1 immunohistochemistry, 3.5-µm-thick sections were prepared from formalin-fixed, paraffin-embedded (FFPE) blocks. The tissue slides were deparaffinized in xylene and then rehydrated through graded ethanol solutions. Antigen was retrieved in 10 mM citrate buffer (pH 6.5) at 110 °C for 10 min. Endogenous peroxidase activity was blocked by incubation in 0.3% hydrogen peroxide for 20 min at room temperature. The slides were then incubated overnight at 4 °C with monoclonal antibody against PD-L1 (a rabbit anti-PD-L1 monoclonal antibody, cat. no. M4424; clone SP142; dilution, 1:100; Spring Bioscience, Pleasanton, CA, USA). Antibody binding was visualized using the ABC peroxidase detection system (Vector Laboratories, Burlingame, CA, USA). Color was developed for PD-L1 immunohistochemistry by incubating the sections in DAB with 0.05% hydrogen peroxide for 2.5 min. Finally, the slides were counterstained with 0.1% hematoxylin for 30 s. Normal human placental tissue was used as a positive control for PD-L1 immunohistochemistry.
PD-L1 expression was evaluated according to the frequency of membrane-stained tumor cells throughout the entire section. As reported previously [17], tumors were considered to have low PD-L1 expression when less than 5% of the cells were stained, and high PD-L1 expression when 5% or more of the cells were stained (Fig. 1c, d).
Assessment of MSI status
To evaluate the MSI status only in immunohistochemically MLH1-negative tumors, DNA was extracted from macro-dissected tissues with negative MLH1 expression using the QIAamp DNA FFPE Tissue Kit (Qiagen). The MSI status was determined using five quasi-monomorphic mononucleotide markers: BAT-25, BAT-26, NR-21, NR24, and MONO-27 (the Promega MSI Analysis System, Promega Corp). Tumors with instability at two or more of the five markers were classified as MSI-H, those with instability at one marker as low microsatellite instability (MSI-L), and those with instability at no markers as microsatellite stable (MSS).
Statistical analysis
We compared clinicopathological factors using the Chi-squared test for categorical variables and the Mann–Whitney U test for continuous variables. Recurrence-free survival (RFS) was defined as the time from the date of surgery to either the date of recurrence or death from any cause. RFS was estimated by the Kaplan–Meier method and tested with the log-rank test. Cox proportional hazards models were used for both univariate and multivariate analyses. A value of P < 0.05 was considered statistically significant. All statistical analyses were performed using the SPSS Statistics software program, version 22 (IBM Corp., Armonk, NY, USA).
Results
Clinicopathological characteristics and MSI status
Of 285 patients, 28 (9.8%) showed negative MLH1 expression and 70 (24.6%) showed high PD-L1 expression. Of 28 immunohistochemically MLH1-negative tumors, 24 (85.7%) were MSI-H and 4 (14.3%) were MSS/MSI-L. Clinicopathological characteristics according to MLH1 and PD-L1 expression are shown in Table 1. More tumors were located in the lower stomach in the MLH1-negative group than in the MLH1-positive group. The presence or absence of PD-L1 expression was not associated with any significant differences between the two groups. In terms of the correlation between MLH1 and PD-L1 expression, the MLH1-negative group had a significantly higher proportion of patients with high PD-L1 expression than the MLH1-positive group (57.1% vs. 21.0%, P < 0.001).
Histological response to preoperative chemotherapy
Of 285 patients, 110 received preoperative chemotherapy; the most frequent regimens were S-1 plus cisplatin plus docetaxel in 37 patients, S-1 plus oxaliplatin plus docetaxel in 29 patients, and S-1 plus cisplatin in 23 patients. Of 110 patients treated with preoperative chemotherapy, 62 (56.4%) and 48 (43.6%) were classified as responders and non-responders, respectively. There was no significant difference in the proportion of responders and non-responders according to the kind of regimens. In terms of the association between histological response and MLH1 expression, the MLH1-negative group had a significantly lower proportion of responders than the MLH1-positive group (16.7% vs. 61.2%, P = 0.005) (Table 2). Meanwhile, there was no significant difference in histological response between the high- and low-PD-L1 expression groups (55.9% vs. 56.6%, P = 0.95).
Recurrence-free survival
We evaluated RFS at the end of the follow-up period, the median duration of which was 58.4 months for all censored patients. In patients without preoperative chemotherapy, RFS in the MLH1-negative group was significantly longer than that in the MLH1-positive group [hazard ratio (HR) 0.30; 95% confidence interval (CI) 0.09–0.95; log-rank P = 0.030] (Fig. 2a). On the other hand, there was no significant difference in RFS between the two groups in the patients with preoperative chemotherapy (HR 0.70; 95% CI 0.30–1.63; log-rank P = 0.41) (Fig. 2b).
There was no significant difference in RFS between the high- and low-PD-L1 expression groups in either the patients without preoperative chemotherapy (HR 0.92; 95% CI 0.52–1.61; log-rank P = 0.76) (Fig. 3a) or those with preoperative chemotherapy (HR 0.79; 95% CI 0.44–1.40; log-rank P = 0.41) (Fig. 3b).
A Cox multivariate analysis of RFS incorporating all potential confounding factors showed that MLH1 expression was an independent prognostic factor, along with pathological T/N status and preoperative chemotherapy (Table 3).
Discussion
This study demonstrated that loss of MLH1 expression was a significant predictive marker of good prognosis and negative response to fluorouracil-based chemotherapy in patients with resectable gastric cancer. There was a large difference in survival between the MLH1-negative and -positive groups in the patients without preoperative chemotherapy but not in those with preoperative chemotherapy. From the negative background of the patients with preoperative chemotherapy who had more advanced tumor stage, this could be explained by the theory that the worse prognosis of the MLH1-positive patients was improved by the preoperative chemotherapy. Meanwhile, PD-L1 expression did not have any predictive characteristics for either prognosis or chemotherapy response.
In this study, we evaluated MLH1 expression as an alternative to MSI status, because it is known that MLH1 is a main component of MMR, and hypermethylation of the MLH1 gene promoter is strongly associated with MSI-H status resulting from MMRD in gastric cancer [7,8,9]. In colorectal cancer, MSI-H is a specific feature of HNPCC resulting from a germline mutation in one of the MMR genes, usually MLH1 or MutS homologue 2 (MSH2) [6]. On the other hand, MSI-H in gastric cancer is rarely associated with HNPCC and is mainly caused by hypermethylation of the MLH1 gene promoter due to H. pylori infection [18]. Indeed, immunohistochemistry findings in this study showed that 24 (85.7%) of 28 MLH1-negative tumors were MSI-H. This result was similar to those of previous studies, which demonstrated that approximately 85% of MLH1-negative gastric cancers were MSI-H [10, 19]. In addition, since immunohistochemistry of MLH1 was shown in a previous report to be a reliable method to detect MMRD and MSI-H [20], MLH1 status evaluated by immunohistochemistry might be a useful alternative to MSI status as a biomarker in gastric cancer.
In the light of the high concordance between MLH1 expression and MSI status in this study, our results are consistent with those of previous studies showing that patients with gastric cancer treated with surgery alone had a significantly better prognosis if their tumors were MSI-H than if they were MSI-L/MSS [21,22,23]. It has been suggested that prognosis is better in patients with MSI-H tumors due to increased lymphocyte infiltration around tumor cells [24,25,26]. MSI-H is a tumor molecular phenotype caused by MMRD, and is characterized by the inability to repair mutations in microsatellite regions. This results in an increased mutation burden, potentially leading to the production of proteins with mutation-associated neoantigens that increase the numbers of anti-tumor lymphocytes around tumors [27,28,29]. In addition, several previous studies in colorectal cancer analyzed the tumor immune microenvironment and demonstrated that tumors with MSI-H exhibited a significantly greater number of cytotoxic lymphocytes, leading to upregulated PD-1 and PD-L1 expression [30, 31].
MSI-H was also reported to be a predictor of negative response to fluorouracil-based chemotherapy in several studies of colorectal cancer and gastric cancer, and thus patients with MSI-L/MSS tumors derived a prognostic benefit from adjuvant fluorouracil-based chemotherapy, whereas patients with MSI-H tumors did not [8,9,10,11, 32]. It was hypothesized that MSI-H results in resistance to fluorouracil by impairing cellular ability to detect DNA damage and activate apoptosis, and more indirectly by increasing the mutation rate throughout the genome [8]. In addition, several studies reported that MLH1-deficient cell lines were relatively resistant to cisplatin, and MSI-H was associated with platinum drug resistance [33, 34]. In this study, all patients were treated with preoperative fluorouracil-based chemotherapy, including mainly cisplatin or oxaliplatin, and thus patients with MLH1-negative tumors might have been unlikely to respond to preoperative chemotherapy.
This study had several limitations. First, it was a retrospective study conducted at a single institution. However, we collected the data of consecutive patients treated with or without preoperative chemotherapy; therefore, we believe that selection bias was minimized. Second, we did not evaluate MLH expression using biopsy samples before preoperative chemotherapy to predict chemotherapy response. Additional studies are required to evaluate whether biopsy samples are clinically useful for predicting the response to preoperative chemotherapy.
In conclusion, our results indicated that MLH1 immunohistochemistry, specifically loss of MLH1 expression, might be a useful alternative to MSI status in terms of predicting a good prognosis and a negative response to chemotherapy in resectable gastric cancer. On the other hand, PD-L1 expression affected neither prognosis nor response to chemotherapy. These results suggest that patients with MLH1-negative gastric cancer should be treated with surgery alone, whereas those with other types of gastric cancer should be treated with a combination of surgery and preoperative or postoperative chemotherapy.
References
Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108.
Kurokawa Y, Doki Y, Mizusawa J, Terashima M, Katai H, Yoshikawa T, et al. Bursectomy versus omentectomy alone for resectable gastric cancer (JCOG1001): a phase 3, open-label, randomised controlled trial. Lancet Gastroenterol Hepatol. 2018;3:460–8.
Colvin H, Mizushima T, Eguchi H, Takiguchi S, Doki Y, Mori M. Gastroenterological surgery in Japan: The past, the present and the future. Ann Gastroenterol Surg. 2017;1:5–10.
Hashimoto T, Kurokawa Y, Mori M, Doki Y. Update on the treatment of gastric cancer. JMA J. 2018;1:40–9.
Cancer Genome Atlas Research Network. Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 2014;513:202–9.
Geiersbach KB, Samowitz WS. Microsatellite instability and colorectal cancer. Arch Pathol Lab Med. 2011;135:1269–77.
Leung SY, Yuen ST, Chung LP, Chu KM, Chan AS, Ho JC. hMLH1 promoter methylation and lack of hMLH1 expression in sporadic gastric carcinomas with high-frequency microsatellite instability. Cancer Res. 1999;59:159–64.
Ribic CM, Sargent DJ, Moore MJ, Thibodeau SN, French AJ, Goldberg RM, et al. Tumor microsatellite-instability status as a predictor of benefit from fluorouracil-based adjuvant chemotherapy for colon cancer. N Engl J Med. 2003;349:247–57.
Sargent DJ, Marsoni S, Monges G, Thibodeau SN, Labianca R, Hamilton SR, et al. Defective mismatch repair as a predictive marker for lack of efficacy of fluorouracil-based adjuvant therapy in colon cancer. J Clin Oncol. 2010;28:3219–26.
Smyth EC, Wotherspoon A, Peckitt C, Gonzalez D, Hulkki-Wilson S, Eltahir Z, et al. Mismatch repair deficiency, microsatellite instability, and survival: an exploratory analysis of the medical research council adjuvant gastric infusional chemotherapy (MAGIC) trial. JAMA Oncol. 2017;3:1197–203.
Choi YY, Kim H, Shin SJ, Kim HY, Lee J, Yang HK, et al. Microsatellite instability and programmed cell death-ligand 1 expression in stage II/III gastric cancer: post hoc analysis of the CLASSIC randomized controlled study. Ann Surg. 2018. https://doi.org/10.1097/SLA.0000000000002803. (Epub ahead of print).
Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372:2509–20.
Muro K, Chung HC, Shankaran V, Geva R, Catenacci D, Gupta S, et al. Pembrolizumab for patients with PD-L1-positive advanced gastric cancer (KEYNOTE-012): a multicentre, open-label, phase 1b trial. Lancet Oncol. 2016;17:717–26.
Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer. 2011;14:101–12.
Kurokawa Y, Shibata T, Sasako M, Sano T, Tsuburaya A, Iwasaki Y, et al. Validity of response assessment criteria in neoadjuvant chemotherapy for gastric cancer (JCOG0507-A). Gastric Cancer. 2014;17(3):514–21.
Seo HM, Chang YS, Joo SH, Kim YW, Park YK, Hong SW, et al. Clinicopathologic characteristics and outcomes of gastric cancers with the MSI-H phenotype. J Surg Oncol. 2009;99:143–7.
Herbst RS, Soria JC, Kowanetz M, Fine GD, Hamid O, Gordon MS, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515:563–7.
Liu JB, Wu XM, Cai J, Zhang JY, Zhang JL, Zhou SH, et al. CpG island methylator phenotype and Helicobacter pylori infection associated with gastric cancer. World J Gastroenterol. 2012;18:5129–34.
Lee HS, Choi SI, Lee HK, Kim HS, Yang HK, Kang GH, et al. Distinct clinical features and outcomes of gastric cancers with microsatellite instability. Mod Pathol. 2002;15:632–40.
Momose K, Yamasaki M, Tanaka K, Miyazaki Y, Makino T, Takahashi T, et al. MLH1 expression predicts the response to preoperative therapy and is associated with PD-L1 expression in esophageal cancer. Oncol Lett. 2017;14:958–64.
Kim SY, Choi YY, An JY, Shin HB, Jo A, Choi H, et al. The benefit of microsatellite instability is attenuated by chemotherapy in stage II and stage III gastric cancer: results from a large cohort with subgroup analyses. Int J Cancer. 2015;137:819–25.
Fang WL, Chang SC, Lan YT, Huang KH, Chen JH, Lo SS, et al. Microsatellite instability is associated with a better prognosis for gastric cancer patients after curative surgery. World J Surg. 2012;36:2131–8.
Marrelli D, Polom K, Pascale V, Vindigni C, Piagnerelli R, De Franco L, et al. Strong prognostic value of microsatellite instability in intestinal type non-cardia gastric cancer. Ann Surg Oncol. 2016;23:943–50.
Fridman WH, Pagès F, Sautès-Fridman C, Galon J. The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer. 2012;12:298–306.
Mlecnik B, Bindea G, Angell HK, Maby P, Angelova M, Tougeron D, et al. Integrative analyses of colorectal cancer show immunoscore is a stronger predictor of patient survival than microsatellite instability. Immunity. 2016;44:698–711.
Jiang Y, Zhang Q, Hu Y, Li T, Yu J, Zhao L, et al. ImmunoScore signature: a prognostic and predictive tool in gastric cancer. Ann Surg. 2018;267:504–13.
Williams DS, Bird MJ, Jorissen RN, Yu YL, Walker F, Zhang HH, et al. Nonsense mediated decay resistant mutations are a source of expressed mutant proteins in colon cancer cell lines with microsatellite instability. PLoS One. 2010;5:e16012.
Rizvi NA, Hellmann MD, Snyder A, Kvistborg P, Makarov V, Havel JJ, et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348:124–8.
McGranahan N, Furness AJ, Rosenthal R, Ramskov S, Lyngaa R, Saini SK, et al. Clonal neoantigens elicit T cell immunoreactivity and sensitivity to immune checkpoint blockade. Science. 2016;351:1463–9.
Phillips SM, Banerjea A, Feakins R, Li SR, Bustin SA, Dorudi S. Tumour-infiltrating lymphocytes in colorectal cancer with microsatellite instability are activated and cytotoxic. Br J Surg. 2004;91:469–75.
Prall F, Duhrkop T, Weirich V, Ostwald C, Lenz P, Nizze H, et al. Prognostic role of CD8+ tumor-infiltrating lymphocytes in stage III colorectal cancer with and without microsatellite instability. Hum Pathol. 2004;35:808–16.
An JY, Kim H, Cheong JH, Hyung WJ, Kim H, Noh SH. Microsatellite instability in sporadic gastric cancer: its prognostic role and guidance for 5-FU based chemotherapy after R0 resection. Int J Cancer. 2012;131:505–11.
Fink D, Nebel S, Aebi S, Zheng H, Cenni B, Nehmé A, et al. The role of DNA mismatch repair in platinum drug resistance. Cancer Res. 1996;56:4881–6.
Aebi S, Fink D, Gordon R, Kim HK, Zheng H, Fink JL, et al. Resistance to cytotoxic drugs in DNA mismatch repair-deficient cells. Clin Cancer Res. 1997;3:1763–7.
Acknowledgements
The authors declare that this study was not funded.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical standards
Written informed consent was obtained from all patients prior to the procedure. The study was approved by the ethics committee of the Institutional Review Board.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hashimoto, T., Kurokawa, Y., Takahashi, T. et al. Predictive value of MLH1 and PD-L1 expression for prognosis and response to preoperative chemotherapy in gastric cancer. Gastric Cancer 22, 785–792 (2019). https://doi.org/10.1007/s10120-018-00918-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10120-018-00918-4