Abstract
Background
Current Japanese gastric cancer treatment guidelines recommend the same endoscopic resection criteria for papillary early gastric cancer (EGC) and well-differentiated (WD) or moderately differentiated (MD) EGC. To evaluate the appropriateness of this recommendation, we compared the clinicopathological characteristics of papillary EGC with those of WD, MD, poorly differentiated (PD), and signet ring cell (SRC) EGC.
Methods
A total of 6710 patients who underwent radical gastrectomy for EGC were included. Clinicopathological characteristics of papillary EGC were retrospectively reviewed and compared with those in other EGC subtypes.
Results
Papillary EGC accounted for 1.9% (130/6710) of total cases. Patients with papillary EGC were older and showed a male predominance compared to patients with PD or SRC EGC. Papillary EGCs showed significantly higher submucosal and lymphovascular invasion rates than WD or MD EGC or PD or SRC EGC. However, the LN metastasis rate of papillary EGC was comparable to or lower than that in other EGC subtypes. LN metastasis rates in mucosal cancers were 1.5%, 1.1%, and 4.0%, and those in submucosal cancers were 9.4%, 11.9%, and 17.6% for papillary EGC, WD or MD EGC, and PD or SRC EGC, respectively. In multivariate analysis, lymphatic invasion and PD or SRC histology were the strongest risk factors for LN metastasis. Among 63 papillary EGC that met the curative endoscopic resection criteria, no case showed LN metastasis.
Conclusions
Endoscopic resection can be indicated for papillary EGC according to current guidelines. Given a considerable lymphovascular invasion rate, careful histological evaluation is required after endoscopic resection for papillary EGC.
Similar content being viewed by others
Introduction
Papillary adenocarcinoma of the stomach is defined as a well-differentiated exophytic gastric carcinoma with elongated finger-like processes lined by cylindrical or cuboidal cells supported by fibromuscular connective tissue cores [1]. As papillary adenocarcinoma is a rare subtype of gastric adenocarcinoma, its clinicopathological characteristics and biological behavior remain unclear [2, 3]. In Japanese classification, papillary adenocarcinoma and well-differentiated (WD) or moderately differentiated (MD) tubular adenocarcinoma are classified together as differentiated-type gastric carcinoma, and the same endoscopic resection criteria are applied in cases of early gastric cancer (EGC) [4]. However, several recent studies have shown that patients with papillary EGC have a higher lymphovascular invasion rate and worse prognosis compared to undifferentiated-type as well as other differentiated-type EGCs [2, 5,6,7,8,9]. This higher lymphovascular invasion rate raised concerns that papillary EGC might have a considerable lymph node (LN) metastasis rate and that current endoscopic resection criteria might be inappropriate for papillary EGC. In fact, recent Japanese and Korean studies reported a higher LN metastasis rate in papillary EGC compared to other subtypes of EGC in univariate analysis [2, 6, 7]. In a Japanese study, however, the papillary component was not an independent risk factor for LN metastasis in EGC [7].
In the present study, we aimed to identify the clinicopathological characteristics of papillary EGC and to evaluate whether papillary EGC is associated with a higher risk of LN metastasis compared to other subtypes of EGC.
Methods
Patients
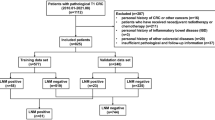
From May 2004 to November 2015, 7573 patients with EGC underwent radical gastrectomy with LN dissection at Samsung Medical Center. Among these patients, the following cases were excluded from the study population: 349 patients with multiple synchronous EGCs, 55 patients with EGC arising from the remnant stomach, 21 patients with missing data, 33 patients with lymphoepithelioma-like carcinoma, 20 patients with mucinous adenocarcinoma, 1 patient with hepatoid adenocarcinoma, and 384 patients undergoing additional surgery after endoscopic resection for submucosal EGC. In our institute, the submucosal invasion depth of the surgical specimen was reported using a three-tier system: SM1, SM2, and SM3 representing invasion of cancer cells into the upper third, middle third, and lower third of the submucosa, respectively [10]. As this system cannot be applied to endoscopic resection specimens that do not contain the whole submucosal layer, we excluded submucosal EGC cases initially treated with endoscopic resection. After exclusions, a total of 6710 patients with EGC were ultimately included in the study population. In Korean and Japanese guidelines [4, 11, 12], EGC consisting of components of both differentiated-type and undifferentiated-type carcinoma [including poorly differentiated (PD) adenocarcinoma, signet ring cell (SRC) carcinoma, and mucinous adenocarcinoma] is classified according to the quantitatively predominant histological type. Accordingly, papillary adenocarcinoma is defined as a tumor in which more than 50% of the tumor area contains papillary structures composed of epithelial projections with a central fibrovascular core as a scaffold in the present study. Clinicopathological data including demographic features, tumor characteristics, and LN metastasis were obtained by the retrospective review of medical records using the intranet resources of Samsung Medical Center. All patients provided informed consent according to our institutional guidelines. The institutional review board of Samsung Medical Center approved the study protocol.
Histopathological evaluation
Specimens were fixed in 10% formalin and then serially sectioned at 5-mm intervals, embedded in paraffin blocks, and stained with hematoxylin and eosin. A tumor was considered as positive for lymphovascular invasion when tumor emboli were found within a space that was clearly lined by endothelial cells. The lymph nodes were cut into two pieces, and the cut surfaces were examined to determine the status of the nodes. Lymph node metastasis was identified using hematoxylin and eosin staining [13].
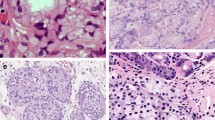
For papillary EGC cases, nuclear atypia grade was determined as low or high grade according to nuclear polymorphism and nuclear polarity observed in cancer cells (Fig. 1) [14]. We also investigated the presence of undifferentiated component in papillary EGC cases.
Statistical analysis
Categorical variables were analyzed using the chi-squared test or Fisher’s exact test. Continuous variables were analyzed using Student’s t test. Survival rates were calculated using the Kaplan–Meier method. To identify independent predictive factors for LN metastasis, multivariate binary logistic regression analysis was performed. The odds ratios and 95% confidence intervals were calculated. A P value less than 0.05 was considered statistically significant.
Results
Clinicopathological characteristics of early gastric papillary adenocarcinoma versus nonpapillary adenocarcinoma
The study population included 130 patients with papillary EGC (1.9%, 130/6710), 2711 patients with WD or MD EGC (40.4%, 2711/6710), and 3869 patients with PD or SRC EGC (57.7%, 3869/6710). The mean age of the study population was 55.8 ± 11.5 years. The proportions of submucosal EGC were 49.2%, 38.2%, and 31.1% for papillary EGC, WD or MD EGC, and PD or SRC EGC, respectively (P < 0.001).
Table 1 summarizes and compares the clinicopathological characteristics of papillary EGC and nonpapillary EGC confined to the mucosal layer. Patients with papillary EGC were older than those with WD or MD EGC or PD or SRC EGC and showed a male predominance compared to those with PD or SRC EGC. Papillary EGCs were larger and more frequently elevated in macroscopic type and showed significantly higher lymphatic and venous invasion rate than WD or MD EGC or PD or SRC EGC. LN metastasis rates in mucosal cancers were 1.5%, 1.1%, and 4.0% for papillary EGC, WD or MD EGC, and PD or SRC EGC, respectively.
Table 2 summarizes and compares the clinicopathological characteristics of papillary EGC and nonpapillary EGC with submucosal invasion. Patients with papillary EGC were older and showed a male predominance compared to patients with PD or SRC EGC. Patients with papillary EGCs more frequently had a macroscopically elevated tumor and showed significantly higher lymphatic and venous invasion rate than those with WD or MD EGC or PD or SRC EGC. LN metastasis rates in submucosal cancers were 9.4%, 11.9%, and 17.6% for papillary EGC, WD or MD EGC, and PD or SRC EGC, respectively.
Predictive factors associated with lymph node metastasis in patients with early gastric cancer
Table 3 shows the results of univariate and multivariate analysis of clinicopathological characteristics associated with LN metastasis in 6710 EGCs. Multivariate analysis revealed that younger age, female sex, lower third tumor location, large tumor size, submucosal invasion, PD adenocarcinoma or SRC carcinoma histology, and the presence of lymphatic invasion were independent predictive factors for LN metastasis. LN metastasis rates in the total study population were 5.4%, 5.2%, and 8.3% for papillary EGC, WD or MD EGC, and PD or SRC EGC, respectively.
In the total study population, lymphatic invasion rates were 26.2%, 13.6%, and 9.9% for papillary EGC, WD or MD EGC, and PD or SRC EGC, respectively. In patients with lymphatic invasion, LN metastasis rates were 14.7%, 26.2%, and 39.3% for papillary EGC, WD or MD EGC, and PD or SRC EGC, respectively. In the total study population, venous invasion rates were 7.7%, 1.6%, and 0.5% for papillary EGC, WD or MD EGC, and PD or SRC EGC, respectively. No papillary EGC patient with venous invasion showed distant metastasis at the time of surgery or during follow-up period. In patients with venous invasion, LN metastasis rates were 10.0%, 27.9%, and 33.3% for papillary EGC, WD or MD EGC, and PD or SRC EGC, respectively.
Clinicopathological characteristics of patients with early gastric papillary adenocarcinoma with lymph node metastasis
Table 4 summarizes the results of univariate analysis of clinicopathological characteristics associated with LN metastasis in 130 papillary EGCs. Papillary EGCs with LN metastasis showed significantly higher rates of tumor size larger than 3.0 cm, deep submucosal invasion, and lymphatic invasion than tumors without LN metastasis. Multivariate analysis could not be performed as the model was not fitted because of the small number of patients with LN metastasis. Among seven papillary EGC patients with LN metastasis (Table 5), six patients (85.7%) had tumors with deep submucosal invasion (SM2 or SM3), and only one patient (14.3%) had mucosal cancer. Tumor size was larger than 3.0 cm in all cases, and lymphatic invasion was present in five cases (71.4%). The mucosal cancer with LN metastasis was 5.5 cm in size and had lymphatic invasion; thus, it did not meet the curative endoscopic resection criteria [4].
Among 130 papillary EGCs, 57 mucosal EGCs and 6 submucosal EGCs met the curative endoscopic resection criteria proposed in the Japanese gastric cancer treatment guidelines [4]. None of these tumors showed LN metastasis in the surgical specimen. The submucosal invasion depth of 6 submucosal EGCs ranged from 50 to 400 µm.
Nuclear atypia grade and presence of undifferentiated component in early gastric papillary adenocarcinoma
We investigated the presence of undifferentiated component and nuclear atypia grade in 118 papillary EGCs for which pathological slides were available. No papillary EGC case showed an undifferentiated component. For nuclear atypia grade, 46 (39.0%) were classified as tumor with low-grade nuclear atypia and 72 (61.0%) as tumor with high-grade nuclear atypia. No significant difference was found in lymphatic invasion and LN metastasis rates between papillary EGCs with low-grade nuclear atypia and high-grade nuclear atypia (Table 6).
Discussion
To achieve curative resection through endoscopic resection, it is critical to select tumors with little risk of LN metastasis. In Korean and Japanese studies, extragastric recurrence rate after curative endoscopic resection for differentiated- and undifferentiated-type EGC ranged from 0.10% to 0.15% [15,16,17,18], which is acceptable considering postoperative morbidity and mortality after radical gastrectomy. Papillary adenocarcinoma is classified as a differentiated-type gastric carcinoma, and current Japanese gastric cancer treatment guidelines recommend the same endoscopic resection criteria for papillary EGC and WD or MD EGC [4]. However, several recent studies have reported that patients with papillary EGC have higher lymphovascular invasion and LN metastasis rate compared to other subtypes of EGC [2, 6, 7], which raises concerns regarding the appropriateness of the current guidelines. In the present study including 6710 EGCs, we found papillary EGC did have a higher lymphovascular invasion rate than WD or MD EGC and PD or SRC EGC. However, the LN metastasis rate in papillary EGC was comparable or lower compared to those in WD or MD EGC or PD or SRC EGC. Furthermore, no papillary EGC showed LN metastasis if it met the curative endoscopic resection criteria. These results support current Japanese gastric cancer treatment guidelines that endoscopic resection can be indicated for papillary EGC, as in WD or MD EGC [4].
Sekiguchi et al. [7] reported results consistent with those from our study. They evaluated whether the presence of a papillary component in more than 10% of the tumor area affected the occurrence of lymphatic invasion or LN metastasis in EGC. In univariate analysis, the presence of a papillary component was significantly associated with both lymphatic invasion and LN metastasis. In multivariate analysis, the presence of a papillary component was identified as an independent risk factor for lymphatic invasion. However, the presence of a papillary component was not an independent risk factor for LN metastasis. Based on these results, they argued that it is unnecessary to change the endoscopic resection criteria for EGC with a papillary component from the current treatment guidelines. Lee et al. [6] compared clinicopathological features of patients with papillary EGC with those in WD or MD EGC and PD, SRC, or mucinous EGC, respectively. Papillary adenocarcinoma is defined as a tumor in which more than 50% of the tumor area contains papillary structures, as in our study. In univariate analysis, they found that the lymphovascular invasion rate in papillary EGC was significantly higher compared to that in nonpapillary EGC. The LN metastasis rate in papillary EGC was also higher than that in nonpapillary EGC, but the difference did not reach statistical significance. Multivariate analysis to identify independent predictive factors for LN metastasis was not performed with the whole study population in their study. When analyzing 56 patients with papillary EGC, lymphatic invasion was the only predictive factor for LN metastasis.
In the present study, both lymphatic invasion and venous invasion rates were the highest in papillary EGC compared to WD or MD EGC and PD or SRC EGC. As lymphovascular invasion is a well-known important risk factor for LN metastasis, it is expected that the LN metastasis rate would be higher in papillary EGC than in other subtypes of EGC. In the present study, however, LN metastasis rate in papillary EGC was comparable or lower than that in WD or MD EGC or PD or SRC EGC in both mucosal and submucosal cancers (1.5%, 1.1%, and 4.0% for mucosal EGCs and 9.45%, 11.9%, and 17.6% for submucosal EGCs, respectively). This mismatch might be explained by the low LN metastasis rates observed in papillary EGC with lymphatic or venous invasion. In the present study, LN metastasis rates in tumors with lymphatic or venous invasion were the lowest in patients with papillary EGC (14.7% and 10.0% in tumors with lymphatic and venous invasion, respectively). Our group previously investigated EGCs with lymphatic invasion and reported that LN metastasis rate was associated with lymphatic invasion grade stratified based on the number of lymphatic tumor emboli in whole sections [19]. Therefore, there might be a difference in lymphatic invasion grade between papillary EGC and other subtypes of EGC with lymphatic invasion.
Nakashima et al. [14] reported that papillary gastric adenocarcinoma with low-grade nuclear atypia was associated with lower frequency of lymphatic or venous invasion and lower frequency of LN metastasis compared to tumors with high-grade nuclear atypia. In their study, 88.7% of papillary EGC was classified as tumor with low-grade nuclear atypia. In our study, however, only 39.0% of papillary EGC was diagnosed as tumor with low-grade nuclear atypia. There was no significant difference in lymphatic invasion and LN metastasis rates between papillary EGCs with low-grade nuclear atypia and high-grade nuclear atypia. Further large studies are necessary to evaluate the role of nuclear atypia grading as a predictor for aggressive tumor features in papillary gastric adenocarcinoma.
In the present study, 57 mucosal papillary EGCs and 6 submucosal papillary EGCs met the curative endoscopic resection criteria, and none of these tumors showed LN metastasis in the surgical specimen. All 7 papillary EGCs with LN metastasis were beyond curative endoscopic resection criteria. In the study by Sekiguchi et al. [7], 4 EGCs with a papillary component in more than 50% of the tumor area showed LN metastasis. All these papillary EGCs had lymphatic invasion and were thus beyond curative endoscopic resection criteria. Consistent with these surgical data, there have been no studies that reported extragastric recurrence after curative endoscopic resection for papillary EGC [15,16,17,18].
This study was limited in that it was performed at a single tertiary referral center and had a retrospective design. Second, despite detailed pathology review on nuclear atypia grade and undifferentiated component in papillary EGCs, we could not clearly identify the mechanism underlying the mismatch between aggressive feature of papillary EGC (high lymphatic invasion, venous invasion, and submucosal invasion rates) and comparable or lower LN metastasis rate in papillary EGC than in other subtypes of EGC.
In conclusion, we found that the LN metastasis rate in papillary EGC was comparable or lower than that in WD or MD EGC or PD or SRC EGC despite its higher lymphovascular invasion rate. Given no LN metastasis in papillary EGC that met the curative endoscopic resection criteria, endoscopic resection can be indicated for papillary EGC according to current guidelines [4]. Careful histological evaluation is required after endoscopic resection for papillary EGC because papillary EGC shows a considerable rate of lymphovascular invasion.
References
Lauwers GY, Carneiro F, Graham DY, Curado MP, Franceschi S, Montgomery E, et al. Gastric carcinoma. In: Bosman FT, Carneiro F, Hruban RH, Theise ND, editors. WHO classification of tumours of the digestive system. Lyon: IARC; 2010. p. 48–58.
Koseki K, Takizawa T, Koike M, Ito M, Nihei Z, Sugihara K. Distinction of differentiated type early gastric carcinoma with gastric type mucin expression. Cancer (Phila). 2000;89:724–32.
Yasuda K, Adachi Y, Shiraishi N, Maeo S, Kitano S. Papillary adenocarcinoma of the stomach. Gastric Cancer. 2000;3:33–8.
Japanese Gastric Cancer A. Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric Cancer. 2017;20:1–19.
Huang Q, Fang C, Shi J, Sun Q, Wu H, Gold JS, et al. Differences in clinicopathology of early gastric carcinoma between proximal and distal location in 438 Chinese patients. Sci Rep. 2015;5:13439.
Lee HJ, Kim GH, Park DY, Kim YK, Jeon HK, Lee BE, et al. Endoscopic submucosal dissection for papillary adenocarcinoma of the stomach: is it really safe? Gastric Cancer. 2017;20:978–86.
Sekiguchi M, Kushima R, Oda I, Suzuki H, Taniguchi H, Sekine S, et al. Clinical significance of a papillary adenocarcinoma component in early gastric cancer: a single-center retrospective analysis of 628 surgically resected early gastric cancers. J Gastroenterol. 2015;50:424–34.
Sekiguchi M, Sekine S, Oda I, Nonaka S, Suzuki H, Yoshinaga S, et al. Risk factors for lymphatic and venous involvement in endoscopically resected gastric cancer. J Gastroenterol. 2013;48:706–12.
Yu H, Fang C, Chen L, Shi J, Fan X, Zou X, et al. Worse prognosis in papillary, compared to tubular, early gastric carcinoma. J Cancer. 2017;8:117–23.
An JY, Baik YH, Choi MG, Noh JH, Sohn TS, Kim S. Predictive factors for lymph node metastasis in early gastric cancer with submucosal invasion: analysis of a single institutional experience. Ann Surg. 2007;246:749–53.
Kim WH, Park CK, Kim YB, Kim YW, Kim HG, Bae HI, et al. A standardized pathology report for gastric cancer. Korean J Pathol. 2005;39:106–13.
Min BH, Kim KM, Park CK, Lee JH, Rhee PL, Rhee JC, et al. Outcomes of endoscopic submucosal dissection for differentiated-type early gastric cancer with histological heterogeneity. Gastric Cancer. 2015;18:618–26.
Pyo JH, Lee H, Min BH, Lee JH, Choi MG, Lee JH, et al. Long-term outcome of endoscopic resection vs. surgery for early gastric cancer: a non-inferiority-matched cohort study. Am J Gastroenterol. 2016;111:240–9.
Nakashima Y, Yao T, Hirahashi M, Aishima S, Kakeji Y, Maehara Y, et al. Nuclear atypia grading score is a useful prognostic factor in papillary gastric adenocarcinoma. Histopathology (Oxf). 2011;59:841–9.
Lee S, Choi KD, Hong SM, Park SH, Gong EJ, Na HK, et al. Pattern of extragastric recurrence and the role of abdominal computed tomography in surveillance after endoscopic resection of early gastric cancer: Korean experiences. Gastric Cancer. 2017;20:843–52.
Min BH, Kim ER, Kim KM, Park CK, Lee JH, Rhee PL, et al. Surveillance strategy based on the incidence and patterns of recurrence after curative endoscopic submucosal dissection for early gastric cancer. Endoscopy. 2015;47:784–93.
Oda I, Oyama T, Abe S, Ohnita K, Kosaka T, Hirasawa K, et al. Preliminary results of multicenter questionnaire study on long-term outcomes of curative endoscopic submucosal dissection for early gastric cancer. Dig Endosc. 2014;26:214–9.
Suzuki H, Oda I, Abe S, Sekiguchi M, Mori G, Nonaka S, et al. High rate of 5-year survival among patients with early gastric cancer undergoing curative endoscopic submucosal dissection. Gastric Cancer. 2016;19:198–205.
Park JW, Ahn S, Lee H, Min BH, Lee JH, Rhee PL, et al. Predictive factors for lymph node metastasis in early gastric cancer with lymphatic invasion after endoscopic resection. Surg Endosc. 2017;31:4419–24.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare they have no conflict of interest.
Human rights statement and informed consent
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964 and later versions. Informed consent to be included in the study, or the equivalent, was obtained from all patients.
Rights and permissions
About this article
Cite this article
Min, BH., Byeon, SJ., Lee, J.H. et al. Lymphovascular invasion and lymph node metastasis rates in papillary adenocarcinoma of the stomach: implications for endoscopic resection. Gastric Cancer 21, 680–688 (2018). https://doi.org/10.1007/s10120-017-0785-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10120-017-0785-7