Abstract
Introduction
Although early detection and successful gastrectomy have improved the survival of patients with gastric cancer, long-term health problems remain troubling. We evaluated the prevalence of osteoporosis and its risk factors in long-term survivors of gastric cancer after gastrectomy.
Methods
We reviewed the medical records of a tertiary hospital between 2007 and 2014 to identify survivors of gastric cancer who had visited our center at around 5 years after gastrectomy. We evaluated their health status, including bone mineral density (BMD). Dual-energy X-ray absorptiometry was used to measure the BMD of the lumbar spine and femur (total and neck area). The prevalence of osteoporosis, defined by a BMD T score <−2.5, was investigated, and clinical variables associated with the presence of osteoporosis were identified.
Results
A total of 250 survivors were included. The mean age was 54.6 years old, and the median follow-up was 6.0 years. The prevalence of osteoporosis was 34.0% (27.4% for men and 43.6% for women). Older age [odds ratio (OR) 5.50, 95% CI 2.33–13.00], higher alkaline phosphatase levels before gastrectomy (OR 5.67, 95% CI 1.36–23.64), and marked weight loss (≥20%) after gastrectomy (OR 3.59, 95% CI 1.32–9.77) were independently associated with the presence of osteoporosis.
Conclusions
In our cohort, osteoporosis was commonly observed in long-term survivors of gastric cancer, and several risk factors for it were identified. To reduce the risk of osteoporosis after gastrectomy, maintaining adequate body weight may be necessary.
Similar content being viewed by others
Introduction
According to the Korea Central Cancer Registry 2013, gastric cancer is the most common cancer in Korea, with the exception of thyroid cancer [1]. Although the incidence of gastric cancer has steadily decreased, it still accounts for an important part of the disease burden of cancer and is the fourth most common cancer worldwide [2]. However, improvements in treatment techniques have enabled early detection and increased the number of long-term gastric cancer survivors [3, 4]. In Korea, the 5-year survival rate of gastric cancer increased from 42.8% in 1993–1995 to 71.5% in 2008–2012, and the 5-year survival rate of early gastric cancer is greater than 90% [1].
Gastrectomy is the best curative treatment for gastric cancer; however, it can cause various health problems, such as weight loss, malnutrition, anemia, and loss of bone density [5, 6]. Osteoporosis, a skeletal disorder predisposing a person to increased fracture risk because of decreased bone strength, is one of the most common complications after gastrectomy [7].
In a previous study, the prevalence of lumbar osteoporosis was 38.3% in Korean gastric cancer patients who had undergone gastrectomy [8]. Another study reported that more than 50% of gastrectomy patients had vertebral fractures with or without osteopenia [9]. The risk of bone loss in gastric cancer patents after gastrectomy is increased by malabsorption, malnutrition, high serum levels of parathyroid hormone (PTH), and cancer therapy [10]. Absorption of calcium and vitamin D is impaired in patients with gastrectomy, because most of the stomach is removed and bypasses such as gastroduodenostomy and gastrojejunostomy are implemented. Thus, gastrectomy may influence the release of gastrin and calcitonin [11,12,13]. Serum levels of gastrin are markedly decreased among patients with subtotal gastrectomy, which induces low gastric acid levels and could interrupt the absorption of dietary calcium. The release of gastrin and calcitonin after meal intake is considerably decreased in patients who have undergone subtotal gastrectomy [13]. Levels of PTH were higher after gastrectomy [14, 15], which would affect bone formation and bone resorption. Cancer therapy, including chemotherapy, could affect bone loss [10, 16]. For instance, 5-fluorouracils such as doxifluridine and cisplatin, which are common chemotherapeutic agents used in gastric cancer, induce apoptosis among osteoblasts and pre-osteoblasts in animal models.
Previous studies have reported a high prevalence of osteoporosis in patients after gastrectomy [8, 9]. An early report by Zittel et al. found a high prevalence of bone disorders after gastrectomy when comparing gastrectomy patients with control subjects [9]. Although that study showed long-term bone metabolic parameters after gastrectomy, it did not demonstrate associated factors affecting bone disorders and included patients with peptic ulcer diseases. Lim et al. reported a high prevalence of osteoporosis in Korean gastric cancer patients after gastrectomy and factors that are associated with osteoporosis [8]. However, in that study, the follow-up period after gastrectomy was about 2–3 years, and thus it could not represent gastric cancer survivors. Therefore, we evaluated the prevalence of osteopenia and osteoporosis among long-term survivors of gastric cancer and the risk factors for low bone density in these patients.
Methods
Study population
We reviewed the medical records of gastric cancer patients referred to the Cancer Prevention Clinic at the Department of Family Medicine of Asan Medical Center (Seoul, Republic of Korea) from January 1, 2009 to December 31, 2014. This study was approved by the institutional review board of Asan Medical Center. The inclusion criteria were survival of at least 5 years after gastrectomy (from January 1, 2004 to December 31, 2008), no evidence of recurrence or metastasis, and the presence of BMD evaluation by dual-energy X-ray absorptiometry (DXA). In total, 270 patients were included in the study. We excluded 3 patients who had already been treated for osteoporosis before being diagnosed with gastric cancer and 11 who had hypercalcemia, hypocalcemia, abnormal liver function tests (levels of AST or ALT that were three times greater than the upper reference value), or impairment of renal function before the operation. Six patients with stage III gastric cancer were excluded. Hence, data from 250 patients were analyzed.
Data collection
From review of the medical records of these patients, we obtained age at gastrectomy, sex, height, weight before and after operation, and treatment records, including tumor stage, operation type, and whether adjuvant chemotherapy had been conducted. Preoperative height and weight were measured when patients were admitted for surgery. Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared. Postoperative weight was measured when patients visited the outpatient clinic for their BMD to be checked. Weight change was calculated using the difference between preoperative and postoperative weight. The percent weight change was calculated using the absolute weight change divided by the preoperative weight. Significant weight loss was defined to be a decrease in weight after gastrectomy of 10% or more. No weight loss was defined to be a decrease in weight less than 10% of preoperative weight or an increasing weight change. Socioeconomic status and health behaviors, including educational level, smoking status, and alcohol consumption, were determined at admission. Smoking status was classified into current smokers, ex-smokers, and never smokers. Alcohol intake was assessed by asking patients whether they usually drank alcoholic beverages. Serum levels of AST, ALT, alkaline phosphatase (ALP; overall ALP value), calcium, and creatinine were checked before gastrectomy. Levels of calcium greater than 10 mg/dl were considered hypercalcemia, and levels less than 8.3 mg/dl were considered hypocalcemia. Levels of creatinine greater than 1.4 mg/dl were considered to indicate impaired renal function.
Measurement of bone mineral density
BMD of the lumbar spine, total femur, and femur neck was measured using DXA (GE Prodigy; Lunar Radiation, Madison, WI, USA) to evaluate the presence of osteoporosis. The BMD of the lumbar spine was evaluated as the average value of L1–L4 and that of the total femur was the average value of four sites in the proximal femur (femoral neck, Ward’s triangle, greater trochanter, and total hip). The BMDs were expressed as absolute values (g/cm2), T scores (compared to young adults), and Z scores (compared to age-matched values) according to Korean reference values provided by the GE-Lunar database. Osteoporosis was defined as a T score less than −2.5 SD, and osteopenia as a T score of −1.0 to −2.5 SD, based on the criteria of the World Health Organization [17]. The BMD of all patients was measured more than 5 years after gastrectomy.
Statistical analysis
Continuous variables are presented as mean ± SD, and categorical variables are presented as numbers with percentages. To compare the characteristics of patients with and without osteoporosis, a chi-squared test or Fisher’s exact test was used for categorical variables, and an independent t test was used for continuous variables. Multiple logistic regression analyses were performed to evaluate the factors associated with osteoporosis. Variables that showed differences in the presence of osteoporosis at a P value <0.1 in univariate associations were selected for multivariate analyses. All analyses were performed using SPSS version 23.0 (IBM SPSS Statistics, Chicago, IL, USA). P values <0.05 were considered statistically significant.
Results
Basic characteristics of the study population
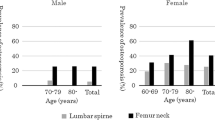
Table 1 presents the basic characteristics of the study population. In total, 250 patients were included in the analyses; 133 (53.2%) were men and 117 (46.8%) were women. The mean age of the patients was 54.6 years, and the median period from gastrectomy was 6.0 years (4.9–18.0 years). Most patients had early (83.2%) and stage I (95.2%) gastric cancer; 80.8% underwent a subtotal gastrectomy (71.2% received Billroth I, and 9.6% received Billroth II), and 13.6% were treated with adjuvant chemotherapy. The average weight before gastrectomy was 68.0 kg in men and 58.1 kg in women; the average weight after gastrectomy was 61.2 kg in men and 52.5 kg in women. Hence, the average weight decreased by 6.3 kg during the follow-up period. The average preoperative ALP was 76.4 IU/l in men and 68.0 IU/l in women. Table 2 lists the BMD values. After gastrectomy, BMDs for the lumbar spine, femur neck, and total femur were 0.987, 0.828, and 0.879 g/cm2, respectively; T scores were −1.470, −0.930, and −0.625, and Z scores were −0.275, 0.230, and 0.264, respectively. All BMD values were higher in men than in women, as shown in Table 2.
Comparison of factors associated with the presence of osteoporosis
Overall, 34.0% of patients had osteoporosis; the prevalence of osteoporosis was 33.2% among early gastric cancer patients and 38.1% among advanced gastric cancer patients. Table 3 provides a descriptive comparison of factors associated with the presence of osteoporosis. Weight before and after gastrectomy was lower in the osteoporosis group; however, the weight change was not significant. Nevertheless, the prevalence of osteoporosis was significantly high for those patients whose body weight was reduced after gastrectomy by at least 20%. In addition, patients in the osteoporosis group were older and had higher pre-operative ALP levels. The classification of early gastric cancer (EGC) or advanced gastric cancer (AGC), gastric cancer stage, and operation type were not associated with osteoporosis in gastric cancer survivors.
Factors associated with osteoporosis after gastrectomy in long-term gastric cancer survivors
Table 4 shows the logistic regression analyses of factors associated with osteoporosis after gastrectomy in long-term gastric cancer survivors. Multivariate logistic regression analyses included factors that had p ≤ 0.1 for comparison of the patients with and without osteoporosis. Among preoperative BMI, postoperative BMI, and the weight before and after gastrectomy, only preoperative BMI was adjusted as a covariate because of multicollinearity. In multivariate analyses, the presence of osteoporosis was associated with older age (OR 5.50, 95% CI 2.33–13.00 in ages ≥60 years old) and high ALP before gastrectomy (OR 5.67, 95% CI 1.36–23.64). The OR of osteoporosis was significantly low in patients with high BMI before gastrectomy (OR 0.82, 95% CI 0.74–0.92) and high in patients who lost at least 20% of their body weight after gastrectomy (OR 3.59, 95% CI 1.32–9.77).
Discussion
We investigated the prevalence of osteoporosis in long-term gastric cancer survivors and identified risk factors associated with osteoporosis. High body weight before and after gastrectomy was a significant protective factor for osteoporosis. In addition, older age and high preoperative serum ALP were associated with a higher prevalence of osteoporosis in these patients.
The prevalence of osteoporosis was greater in long-term gastric cancer survivors than in the general population in Korea. In the Korean National Health and Nutritional Examination Survey (KNHNES), the prevalence of osteoporosis was 21.8% in persons 50 years old or older, 7.8% in men and 34.9% in women [18]. In our study, the prevalence was 34.0% in all patients, 27.4% in men and 43.6% in women. It is notable that the prevalence of male osteoporosis in our study was more than three times as high as in the KNHNES. Although direct comparison with our study is impossible, an earlier study on gastrectomy patients found that almost 55% of patients had osteopenia or vertebral fractures [9]. In a previous study of Korean gastric cancer patients, the prevalence of osteoporosis was 39.6% (28.9% in males and 54.0% in females) at the lumbar spine [8], which was a slightly higher prevalence than that found in our study. However, that study included patients with advanced stages of gastric cancer (stage III and stage IV) and a greater average age (63.9 years old) than the participants in our study.
Body weight is one of the most significant factors associated with osteoporosis [19, 20]. A low body weight may decrease mechanical loading on weight-bearing bones, such as the hip bone and the spine [19]. Furthermore, low levels of parathyroid hormone and extra-ovarian estrogen may increase calcium loss and decrease BMD [20]. In our study, body weight of the patients in the osteoporosis group was significantly lower both before and after gastrectomy, and this association remained significant in multivariate analyses. On the other hand, weight change was not associated with the prevalence of osteoporosis. As we did not evaluate preoperative BMD, we were unable to investigate any potential associations between the loss of BMD and weight loss. However, considering that peak bone mass is formed in early life [21], body weight before gastrectomy may have a more significant effect on the development of osteoporosis than body weight changes after gastrectomy.
High ALP levels before gastrectomy were also associated with osteoporosis. ALP is a bone turnover marker that is inversely correlated with BMD in the general population. High ALP levels are associated with osteoporotic diseases such as primary hyperparathyroidism [22]. Although we did not measure additional bone-turnover markers, the role of ALP in BMD may not differ between those with and without gastrectomy.
The type of operation and time from gastrectomy were not associated with osteoporosis in our study, in line with previous studies [8, 23]. Thus, the risk of osteoporosis may be associated with gastrectomy itself rather than the surgical method. The American Gastroenterological Association stated in 2003 that patients who had undergone gastrectomy 10 or more years previously should receive a DXA examination [24]. According to our results, the prevalence of osteoporosis was high even in patients who had been followed up for less than 10 years, and it was not associated with time since gastrectomy. Because we did not compare BMDs before and after gastrectomy, it is still unclear whether time since gastrectomy is a risk factor for osteoporosis in long-term gastric cancer survivors. In conclusion, age, body weight, and ALP level before gastrectomy were the relevant factors for osteoporosis, not the period from gastrectomy.
The recommendations for follow-up schedules, treatment, and prevention for osteoporosis after gastrectomy are not well defined among gastric patients in the current guidelines. Recent studies have reported a risk of bone loss and low vitamin D levels among obese patients who have had bariatric surgery and have suggested evaluating vitamin D level, monitoring BMD, and supplementing with oral calcium (800–1200 mg/day). However, because these recommendations might not apply to gastric cancer patients who undergo gastrectomy, specific recommendations are necessary for them.
There were several limitations in our study. First, we retrospectively included gastric cancer patients whose BMDs were examined at a single institution. Therefore, our study does not represent all gastric cancer patients who undergo gastrectomy, and the practice guideline for DXA was not clearly defined in the medical records. Second, although we considered that several factors might influence the development of osteoporosis, we could not adjust for all possible covariates, such as calcium and/or vitamin D intake, comorbidities affecting BMD, fracture history, and physical activity. Third, patients with elevated ALP levels did not receive further examination to evaluate parathyroid or hepatobiliary diseases. Last, medication prescribed at other hospitals was not fully determined, particularly for medication affecting BMD, such as calcium and vitamin D supplements, bisphosphonates, and steroids. Despite these limitations, our study had a long follow-up period and was able to estimate the long-term effects of gastrectomy. In addition, a sufficient number of gastric cancer survivors was included in our analyses. Because we followed a single hospital’s protocol, variations in surveillance protocol and BMD measurement are less likely to exist.
Conclusions
The prevalence of osteoporosis is higher in long-term gastric cancer survivors who have undergone gastrectomy than in the general population. Older age, low body weight before and after gastrectomy, and increased serum levels of ALP were associated with osteoporosis, although changes in body weight and period from gastrectomy were not. Hence, BMD should be evaluated in long-term gastric cancer survivors.
References
Cancer Registration Statistics. [cited 2016 October 20]. 2013. http://kosis.kr/eng/statisticsList/statisticsList_01List.jsp?vwcd=MT_ETITLE&parmTabId=M_01_01#SubCont. Accessed 15 Nov 2017.
Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108. https://doi.org/10.3322/caac.21262 (Epub 2015/02/06; PubMed PMID:25651787).
Lee H-J, Yang H-K, Ahn Y-O. Gastric cancer in Korea. Gastric Cancer. 2002;5(3):0177–82. https://doi.org/10.1007/s101200200031.
Jung KW, Won YJ, Kong HJ, Oh CM, Cho H, Lee DH, et al. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2012. Cancer Res Treat. 2015;47(2):127–41. https://doi.org/10.4143/crt.2015.060 (PubMed PMID:25761484; PubMed Central PMCID:PMC4398120).
Liedman B. Symptoms after total gastrectomy on food intake, body composition, bone metabolism, and quality of life in gastric cancer patients–is reconstruction with a reservoir worthwhile? Nutrition. 1999;15(9):677–82 (Epub 1999/09/01; PubMed PMID:10467612).
Jun JH, Yoo JE, Lee JA, Kim YS, Sunwoo S, Kim BS, et al. Anemia after gastrectomy in long-term survivors of gastric cancer: a retrospective cohort study. Int J Surg (Lond). 2016;28:162–8. https://doi.org/10.1016/j.ijsu.2016.02.084 (Epub 2016/03/05; PubMed PMID:26931339).
Lim JS, Lee J-I. Prevalence, pathophysiology, screening and management of osteoporosis in gastric cancer patients. J Gastric Cancer. 2011;11(1):7–15.
Lim JS, Kim SB, Bang HY, Cheon GJ, Lee JI. High prevalence of osteoporosis in patients with gastric adenocarcinoma following gastrectomy. World J Gastroenterol. 2007;13(48):6492–7 (Epub 2007/12/29; PubMed PMID:18161918).
Zittel TT, Zeeb B, Maier GW, Kaiser GW, Zwirner M, Liebich H, et al. High prevalence of bone disorders after gastrectomy. Am J Surg. 1997;174(4):431–8 (Epub 1997/10/23; PubMed PMID:9337169.
Lim JS, Lee JI. Prevalence, pathophysiology, screening and management of osteoporosis in gastric cancer patients. J Gastric Cancer. 2011;11(1):7–15. https://doi.org/10.5230/jgc.2011.11.1.7 (Epub 2011/11/15; PubMed Central PMCID:PMCPMC3204475).
Heiskanen JT, Kroger H, Paakkonen M, Parviainen MT, Lamberg-Allardt C, Alhava E. Bone mineral metabolism after total gastrectomy. Bone (NY). 2001;28(1):123–7 (Epub 2001/02/13; PubMed PMID:11165953).
Kwon SJ, Hahm JS, Cho YJ, Ahn Y, Shin DI. The influence of gastrectomy on the change of bone metabolism and bone density. Korean J Intern Med. 2000;15(1):25–31.
Filipponi P, Gregorio F, Cristallini S, Mannarelli C, Blass A, Scarponi AM, et al. Partial gastrectomy and mineral metabolism: effects on gastrin-calcitonin release. Bone Miner. 1990;11(2):199–208 (Epub 1990/11/01; PubMed PMID:2268747.
Baek KH, Jeon HM, Lee SS, Lim DJ, Oh KW, Lee WY, et al. Short-term changes in bone and mineral metabolism following gastrectomy in gastric cancer patients. Bone (NY). 2008;42(1):61–7. https://doi.org/10.1016/j.bone.2007.08.027 (PubMed PMID:17942383).
Maier GW, Kreis ME, Zittel TT, Becker HD. Calcium regulation and bone mass loss after total gastrectomy in pigs. Ann Surg. 1997;225(2):181–92 (Epub 1997/02/01. PubMed PMID:9065295; PubMed Central PMCID:PMCPMC1190647).
Stava CJ, Jimenez C, Hu MI, Vassilopoulou-Sellin R. Skeletal sequelae of cancer and cancer treatment. J Cancer Surviv Res Pract. 2009;3(2):75–88. https://doi.org/10.1007/s11764-009-0083-4 (Epub 2009/05/05; PubMed PMID:19412668).
Genant HK, Cooper C, Poor G, Reid I, Ehrlich G, Kanis J, et al. Interim report and recommendations of the World Health Organization Task-Force for Osteoporosis. Osteoporos Int. 1999;10(4):259–64 (Epub 2000/02/29; PubMed PMID:10692972).
Korea Health Statistics. Korea National Health and Nutrition Examination Survey (KNHANES V-1). 2010. [cited 2013 Aug 8]. https://knhanes.cdc.go.kr/knhanes/eng/index.do. Accessed 15 Nov 2017.
Villareal DT, Fontana L, Weiss EP, Racette SB, Steger-May K, Schechtman KB, et al. Bone mineral density response to caloric restriction–induced weight loss or exercise-induced weight loss: a randomized controlled trial. Arch Intern Med. 2006;166(22):2502–10.
Shapses SA, Riedt CS. Bone, body weight, and weight reduction: what are the concerns? J Nutr. 2006;136(6):1453–6 (Epub 2006/05/17; PubMed PMID:16702302; PubMed Central PMCID:PMCPmc4016235).
Sopher AB, Fennoy I, Oberfield SE. An update on childhood bone health: mineral accrual, assessment and treatment. Curr Opin Endocrinol Diab Obesity. 2015;22(1):35–40. https://doi.org/10.1097/med.0000000000000124 (Epub 2014/12/18; PubMed PMID:25517023; PubMed Central PMCID:PMCPMC4405140).
Biver E, Chopin F, Coiffier G, Brentano TF, Bouvard B, Garnero P, et al. Bone turnover markers for osteoporotic status assessment? A systematic review of their diagnosis value at baseline in osteoporosis. Joint Bone Spine. 2012;79(1):20–5. https://doi.org/10.1016/j.jbspin.2011.05.003 (Epub 2011/07/05; PubMed PMID:21724445).
Adachi Y, Shiota E, Matsumata T, Iso Y, Yoh R, Kitano S. Osteoporosis after gastrectomy: bone mineral density of lumbar spine assessed by dual-energy X-ray absorptiometry. Calcif Tissue Int. 2000;66(2):119–22 (Epub 2000/02/01; PubMed PMID:10652959).
Kahrilas PJ, Shaheen NJ, Vaezi MF. American Gastroenterological Association medical position statement: guidelines on osteoporosis in gastrointestinal diseases. Gastroenterology. 2003;124(3):791–4. https://doi.org/10.1053/gast.2003.50107 (Epub 2003/03/04; PubMed PMID:12612916).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Nothing to declare.
Research involving human participants and/or animals
Human participants were involved.
Informed consent
This study was approved by the Institutional Review Board of Asan Medical Center (2016-0125); the need for informed consent was waived because the analysis was retrospective.
Rights and permissions
About this article
Cite this article
Yoo, S.H., Lee, J.A., Kang, S.Y. et al. Risk of osteoporosis after gastrectomy in long-term gastric cancer survivors. Gastric Cancer 21, 720–727 (2018). https://doi.org/10.1007/s10120-017-0777-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10120-017-0777-7