Abstract
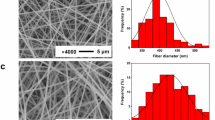
Gelatin, a natural proteinous polymer, was used to co-electrospin with poly(butylene succinate) (PBS) in order to improve the mechanical properties of PBS membrane and facilitate its applications in biomedical field. The PBS/gelatin blend membranes have narrower distribution of fiber diameter and smoother surface than neat PBS membrane. The contact angles, water absorption rates and water uptakes of the PBS/gelatin blend membranes were measured, showing increased hydrophilicity. The interaction between PBS and gelatin was investigated by attenuated total reflectance Fourier transform infrared spectroscopy (ATR-FTIR) and differential scanning calorimetry (DSC). The mechanical properties of PBS/gelatin blend membranes in both dry and wet states were evaluated by uniaxial tensile tests. In the dry state, the PBS/gelatin blend membrane containing 10% gelatin has a 3-times increase in tensile strength without any adverse effect on ductility because of the existence of interaction between the two blend components, little change in crystallinity of PBS, and possible interaction between any adjacent fibers; the tensile strength and elongation at break are even better in the wet state attributed to some gelatin on fiber surfaces, which act as a binder in the presence of water. The potential applications of PBS/gelatin blend membranes were demonstrated by successful immobilization of thrombin, a clinically-used hemostatic drug. The thrombin-loaded membrane could be used for rapid hemostasis.
Similar content being viewed by others
References
Li, H.; Chang, J.; Cao, A.; Wang, J. In vitro evaluation of biodegradable poly(butylene succinate) as a novel biomaterial. Macromol. Biosci. 2005, 5(5), 433–440.
Jeong, E. H.; Im, S. S.; Youk, J. H. Electrospinning and structural characterization of ultrafine poly(butylene succinate) fibers. Polymer 2005, 46(23), 9538–9543.
Xu, J.; Guo, B. H. Poly(butylene succinate) and its copolymers: Research, development and industrialization. Biotechnol. J. 2010, 5(11), 1149–1163.
Wang, X. W.; Zhang, C. A.; Wang, P. L.; Zhao, J.; Zhang, W.; Ji, J. H.; Hua, K.; Zhou, J.; Yang, X. B.; Li, X. P. Enhanced performance of biodegradable poly(butylene succinate)/graphene oxide nanocomposites via in situ polymerization. Langmuir 2012, 28(18), 7091–7095.
Tian, L.; Wang, P.; Zhao, Z.; Ji, J. Antimicrobial activity of electrospun poly(butylenes succinate) fiber mats containing PVP-capped silver nanoparticles. Appl. Biochem. Biotechnol. 2013, 171(7), 1890–1899.
Kimble, L. D.; Bhattacharyya, D. In vitro degradation effects on strength, stiffness, and creep of PLLA/PBS: a potential stent material. Int. J. Polym. Mater. Polym. Biomater. 2015, 64(6), 299–310.
Liu, Y.; He, J. H.; Yu, J. Y. Preparation and morphology of poly(butylene succinate) nanofibers via electrospinning. Fibres Text. East. Eur. 2007, 15(4), 30–33.
San. Z. X.; Li. C. J. Study on soakage control of PBS nanofibers by electrospinning. New Chem. Mater 2007, 35(2), 63–65.
Su, Z.; Ding, J.; Wei, G. Electrospinning: a facile technique for fabricating polymeric nanofibers doped with carbon nanotubes and metallic nanoparticles for sensor applications. RSC Adv 2014, 4(94), 52598–52610.
Zhang, M.; Zhao, X.; Zhang, G.; Wei, G.; Su, Z. Electrospinning design of functional nanostructures for biosensor applications. J. Mater. Chem. B 2017, 5(9), 1699–1711.
Abrigo, M.; McArthur, S. L.; Kingshott, P. Electrospun nanofibers as dressings for chronic wound care: advances, challenges, and future prospects. Macromol. Biosci. 2014, 14(6), 772–792.
Hu, X.; Liu, S.; Zhou, G.; Huang, Y.; Xie, Z.; Jing, X. Electrospinning of polymeric nanofibers for drug delivery applications. J. Control. Release 2014, 185(27), 12–21.
Wang, Z. G.; Wan, L. S.; Liu, Z. M.; Huang, X. J.; Xu, Z. K. Enzyme immobilization on electrospun polymer nanofibers: an overview. J. Mol. Catal. B Enzym. 2009, 56(4), 189–195.
Rieger, K. A.; Birch, N. P.; Schiffman, J. D. Designing electrospun nanofiber mats to promote wound healing—a review. J. Mater. Chem. B 2013, 1(36), 4531–4541.
Liu, Y. M.; Li, Q.; Liu, H. H.; Cheng, H. H.; Yu, J.; Guo, Z. X. Antibacterial thermoplastic polyurethane electrospun fiber mats prepared by 3-aminopropyltriethoxysilane-assisted adsorption of Ag nanoparticles. Chinese J. Polym. Sci. 2017, 35(6), 713–720.
Liu, H. H.; Li, Q.; Liang, X.; Xiong, X.; Yu, J.; Guo, Z. X. Antibacterial polycaprolactone electrospun fiber mats prepared by soluble eggshell membrane protein–assisted adsorption of silver nanoparticles. J. Appl. Polym. Sci. 2016, 133(35), 43850.
Ma, L. C.; Wang, J. N.; Li, L.; Li, C. J. Preparation of PET/CTS antibacterial composites nanofiber membranes Used for air filter by electrospinning. Acta Polymerica Sinica (in Chinese) 2015, (2), 3–227.
Xu, T.; Yang, H. Y.; Yang, D. Z.; Yu, Z. Z. Polylactic acid nanofiber scaffold decorated with chitosan islandlike topography for bone tissue engineering. ACS Appl. Mater. Interfaces 2017, 9(25), 21094–21104.
Arras, M. M. L.; Jana, R.; Mühlstädt, M.; Maenz, S.; Andrews, J.; Su, Z.; Grasl, C.; Jandt, K. D. In situ formation of nanohybrid shish-kebabs during electrospinning for the creation of hierarchical shish-kebab structures. Macromolecules 2016, 49(9), 3550–3558.
Cheng, H. H.; Xiong, J.; Xie, Z. N.; Zhu, Y. T.; Liu, Y. M.; Wu, Z. Y.; Yu, J.; Guo, Z. X. Thrombin-loaded poly(butylene succinate)-based electrospun membranes for rapid hemostatic application. Macromol. Mater. Eng. DOI: 10.1002/mame.201700395.
Li, Y.; Zhang, P.; Ouyang, Z.; Zhang, M.; Lin, Z.; Li, J.; Su, Z.; Wei, G. Nanoscale graphene doped with highly dispersed silver nanoparticles: quick synthesis, facile fabrication of 3D membrane-modifi ed electrode, and super performance for electrochemical sensing. Adv. Funct. Mater. 2016, 26, 3–2134.
Zhang, P.; Zhao, X.; Ji, Y.; Ouyang, Z.; Wen, X.; Li, J.; Su, Z.; Wei, G. Electrospinning graphene quantum dots into a nanofibrous membrane for dual-purpose fluorescent and electrochemical biosensors. J. Mater. Chem. B 2015, 3, 3–2496.
Su, Z.; Li, J.; Ouyang, Z.; Matthias, M. L. A.; Wei, G.; Klaus, D. Biomimetic 3D hydroxyapatite architectures with interconnected pores based on electrospun biaxially orientated PCL nanofibers. RSC Adv 2014, 4, 3–14839.
Li, X.; Xie, J.; Yuan, X.; Xia, Y. Coating electrospun poly(ε-caprolactone) fibers with gelatin and calcium phosphate and their use as biomimetic scaffolds for bone tissue engineering. Langmuir 2008, 24(24), 14145–14150.
Shi, R.; Geng, H.; Gong, M.; Ye, J.; Wu, C.; Hu, X.; Zhang, L. Long-acting and broad-spectrum antimicrobial electrospun poly(ε-caprolactone)/gelatin micro/nanofibers for wound dressing. J. Colloid Interface Sci. 2018, 509, 3–284.
Behrens, A. M.; Sikorski, M. J.; Kofinas, P. Hemostatic strategies for traumatic and surgical bleeding. J. Biomed. Mater. Res. A 2014, 102(11), 4182–4194.
Hwang, P. T. J.; Murdock, K.; Alexander, G. C.; Salaam, A. D.; Ng, J. I.; Lim, D. J.; Dean, D.; Jun, H. W. Poly(ε-caprolactone)/gelatin composite electrospun scaffolds with porous crater-like structures for tissue engineering. J. Biomed. Mater. Res. A 2016, 104(4), 1017–1029.
Meng, Z. X.; Wang, Y. S.; Ma, C.; Zheng, W.; Li, L.; Zheng, Y. F. Electrospinning of PLGA/gelatin randomly-oriented and aligned nanofibers as potential scaffold in tissue engineering. Mater. Sci. Eng. C 2010, 30(8), 1204–1210.
Dhandayuthapani, B.; Krishnan, U. M.; Sethuraman, S. Fabrication and characterization of chitosan-gelatin blend nanofibers for skin tissue engineering. J. Biomed. Mater. Res. B Appl. Biomater. 2010, 94(1), 264–272.
Kim, S. E.; Heo, D. N.; Lee, J. B.; Kim, J. R.; Park, S. H.; Jeon, S. H.; Kwon, I. K. Electrospun gelatin/polyurethane blended nanofibers for wound healing. Biomed. Mater. 2009, 4(4), 044106.
Jamadi, E. S.; Ghasemi Mobarakeh, L.; Morshed, M.; Sadeghi, M.; Prabhakaran, M. P.; Ramakrishna, S. Synthesis of polyester urethane urea and fabrication of elastomeric nanofibrous scaffolds for myocardial regeneration. Mater. Sci. Eng. C 2016, 63, 3–116.
Lee, J.; Tae, G.; Kim, Y. H.; Park, I. S.; Kim, S.H.; Kim, S. H. The effect of gelatin incorporation into electrospun poly(Llactide-co-ε-caprolactone) fibers on mechanical properties and cytocompatibility. Biomaterials 2008, 29(12), 1872–1879.
Yan, S.; Xiaoqiang, L.; Shuiping, L.; Hongsheng, W.; Chuanglong, H. Fabrication and properties of PLLA-gelatin nanofibers by electrospinning. J. Appl. Polym. Sci. 2010, 117(1), 542–547.
Yao, R.; He, J.; Meng, G.; Jiang, B.; Wu, F. Electrospun PCL/gelatin composite fibrous scaffolds: mechanical properties and cellular responses. J. Biomater. Sci. Polym. Ed. 2016, 27(9), 824–838.
Xiong, X.; Li, Q.; Lu, J. W.; Guo, Z. X.; Sun, Z. H.; Yu, J. Fibrous scaffolds made by co-electrospinning soluble eggshell membrane protein with biodegradable synthetic polymers. J. Biomater. Sci.-Polym. Ed. 2012, 23(9), 1217–1231.
Mao, B.; Liu, B.; Wang, Y. F.; Li, G. N.; Song, Y. Z.; Ma, L. P.; Liu, G. H. Preparation of Au colloid of small size in aqueous solution. Rare Met. Mater. Eng. 2009, 38(3), 515–518.
Zhang, X. C.; Xiong, X.; Yu, J.; Guo, Z. X. Aminefunctionalized thermoplastic polyurethane electrospun fibers prepared by co-electrospinning with 3- aminopropyltriethoxysilane and preparation of conductive fiber mats. Polymer 2012, 53(22), 5190–5196.
Cheng, H. H.; Chen, F.; Yu, J.; Guo, Z. X. Gold-nanoparticledecorated thermoplastic polyurethane electrospun fibers prepared through a chitosan linkage for catalytic applications. J. Appl. Polym. Sci. 2016, 133, 44336.
Xiong, X.; Li, Q.; Zhang, X. C.; Yu, J.; Guo, Z. X. Preparation, characterization and application of amine-functionalized poly(lactic acid) electrospun fibers. Chem. J. Chinese Universities 2014, 35(6), 1323–1329.
Rong, J.; Liang, M.; Xuan, F.; Sun, J.; Zhao, L.; Zhen, H.; Tian, X.; Liu, D.; Zhang, Q.; Peng, C.; Yao, T.; Li, F.; Wang, X.; Han, Y.; Yu, W. Alginate-calcium microsphere loaded with thrombin: A new composite biomaterial for hemostatic embolization. Int. J. Biol. Macromol. 2015, 75, 479–488.
Acknowledgments
We thank Technical Institute of Physics and Chemistry, Chinese Academy of Sciences, for kindly providing the PBS. This work was financially supported by the National Natural Science Foundation of China (No. 51173093) and Clinical Innovation Program of Chinese PLA General Hospital (No. 2012FC-ZHCG- 4001).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, L., Cheng, HH., Xiong, J. et al. Improved Mechanical Properties of Poly(butylene succinate) Membrane by Co-electrospinning with Gelatin. Chin J Polym Sci 36, 1063–1069 (2018). https://doi.org/10.1007/s10118-018-2112-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10118-018-2112-0