Abstract
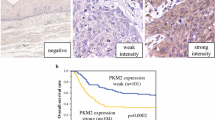
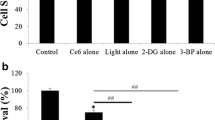
To investigate whether the Warburg effect is a key modulator on the resistance mechanism of photodynamic therapy (PDT). Glycolysis was examined by the test of lactate product and glucose uptake at different post-PDT time points. Cell viability was detected by the CCK-8 assay and cell proliferation was detected by colony formation assay. The expression of glycolysis and related proteins were examined by western blotting. Target gene was silenced by RNAi. In the present study, we assessed the effect of PDT on cancer cell glycolysis. Our team has demonstrated that pyruvate kinase M2 (PKM2), a key speed-limiting enzyme of glycolysis, was significantly overexpressed in patients with esophageal cancer. Our results in the present study showed that PKM2 was downregulated, and lactate product and glucose uptake were inhibited in cells exposed to 5-aminolevulinic acid (5-ALA)–mediated PDT at 4 h after treatment. However, at 24 h after PDT, we observed a substantial increase in PKM2 expression, lactate product, and glucose uptake. Moreover, silencing of PKM2 gene abrogated the upregulatory effect of PDT on glycolysis at late post-PDT period. 2-Deoxy-D-glucose (2-DG) is a recognized chemical inhibitor of glycolysis. The combined treatment of 2-DG and PDT significantly inhibited tumor growth in vitro at 24 h. These results demonstrate that PDT drives the Warburg effect in a time-dependent manner, and PKM2 plays an important role in this progress, which indicated that PKM2 may be a potential molecular target to increase the sensitivity of esophageal cancer cells to PDT.





Similar content being viewed by others
References
Bollschweiler E, Plum P, Monig SP, Holscher AH (2017) Current and future treatment options for esophageal cancer in the elderly. Expert Opin Pharmacother 18(10):1001–1010. https://doi.org/10.1080/14656566.2017.1334764
Kato H, Nakajima M (2013) Treatments for esophageal cancer: a review. Gen Thorac Cardiovasc Surg 61(6):330–335. https://doi.org/10.1007/s11748-013-0246-0
Yoshioka M, Ohashi S, Ida T, Nakai Y, Kikuchi O, Amanuma Y, Matsubara J, Yamada A, Miyamoto S, Natsuizaka M, Nakagawa H, Chiba T, Seno H, Muto M (2017) Distinct effects of EGFR inhibitors on epithelial- and mesenchymal-like esophageal squamous cell carcinoma cells. J Exp Clin Cancer Res 36(1):101. https://doi.org/10.1186/s13046-017-0572-7
Li Y, Sui H, Jiang C, Li S, Han Y, Huang P, Du X, Du J, Bai Y (2018) Dihydroartemisinin increases the sensitivity of photodynamic therapy via NF-kappaB/HIF-1alpha/VEGF pathway in esophageal cancer cell in vitro and in vivo. Cell Physiol Biochem 48(5):2035–2045. https://doi.org/10.1159/000492541
Li YJ, Zhou JH, Du XX, de X J, Wu CL, Huang P, Han Y, Sui H, Wei XL, Liu L, Yuan HH, Zhang TT, Zhang WJ, Xie R, Lang XH, Liu T, Jiang CL, Wang LY, Bai YX (2014) Dihydroartemisinin accentuates the anti-tumor effects of photodynamic therapy via inactivation of NF-kappaB in Eca109 and Ec9706 esophageal cancer cells. Cell Physiol Biochem 33(5):1527–1536. https://doi.org/10.1159/000358716
Muchowicz A, Wachowska M, Stachura J, Tonecka K, Gabrysiak M, Wolosz D, Pilch Z, Kilarski WW, Boon L, Klaus TJ, Golab J (2017) Inhibition of lymphangiogenesis impairs antitumour effects of photodynamic therapy and checkpoint inhibitors in mice. Eur J Cancer 83:19–27. https://doi.org/10.1016/j.ejca.2017.06.004
Abo-Zeid MAM, Abo-Elfadl MT, Mostafa SM (2018) Photodynamic therapy using 5-aminolevulinic acid triggered DNA damage of adenocarcinoma breast cancer and hepatocellular carcinoma cell lines. Photodiagn Photodyn Ther 21:351–356. https://doi.org/10.1016/j.pdpdt.2018.01.011
Kuzyniak W, Schmidt J, Glac W, Berkholz J, Steinemann G, Hoffmann B, Ermilov EA, Gurek AG, Ahsen V, Nitzsche B, Hopfner M (2017) Novel zinc phthalocyanine as a promising photosensitizer for photodynamic treatment of esophageal cancer. Int J Oncol 50(3):953–963. https://doi.org/10.3892/ijo.2017.3854
Maas AL, Carter SL, Wileyto EP, Miller J, Yuan M, Yu G, Durham AC, Busch TM (2012) Tumor vascular microenvironment determines responsiveness to photodynamic therapy. Cancer Res 72(8):2079–2088. https://doi.org/10.1158/0008-5472.Can-11-3744
Yi E, Yang CK, Leem C, Park Y, Chang JE, Cho S, Jheon S (2014) Clinical outcome of photodynamic therapy in esophageal squamous cell carcinoma. J Photochem Photobiol B 141:20–25. https://doi.org/10.1016/j.jphotobiol.2014.09.001
Buttar NS, Wang KK, Lutzke LS, Krishnadath KK, Anderson MA (2001) Combined endoscopic mucosal resection and photodynamic therapy for esophageal neoplasia within Barrett’s esophagus. Gastrointest Endosc 54(6):682–688. https://doi.org/10.1067/gien.2001.0003
Olsen CE, Weyergang A, Edwards VT, Berg K, Brech A, Weisheit S, Hogset A, Selbo PK (2017) Development of resistance to photodynamic therapy (PDT) in human breast cancer cells is photosensitizer-dependent: possible mechanisms and approaches for overcoming PDT-resistance. Biochem Pharmacol 144:63–77. https://doi.org/10.1016/j.bcp.2017.08.002
Wan J, Wu W, Che Y, Kang N, Zhang R (2015) Low dose photodynamic-therapy induce immune escape of tumor cells in a HIF-1alpha dependent manner through PI3K/Akt pathway. Int Immunopharmacol 28(1):44–51. https://doi.org/10.1016/j.intimp.2015.05.025
Rodriguez ME, Catrinacio C, Ropolo A, Rivarola VA, Vaccaro MI (2017) A novel HIF-1alpha/VMP1-autophagic pathway induces resistance to photodynamic therapy in colon cancer cells. Photochem Photobiol Sci 16(11):1631–1642. https://doi.org/10.1039/c7pp00161d
Xu J, Li J, Yu Z, Rao H, Wang S, Lan H (2017) HMGB1 promotes HLF-1 proliferation and ECM production through activating HIF1-alpha-regulated aerobic glycolysis. Pulm Pharmacol Ther 45:136–141. https://doi.org/10.1016/j.pupt.2017.05.015
Kim JW, Tchernyshyov I, Semenza GL, Dang CV (2006) HIF-1-mediated expression of pyruvate dehydrogenase kinase: a metabolic switch required for cellular adaptation to hypoxia. Cell Metab 3(3):177–185. https://doi.org/10.1016/j.cmet.2006.02.002
Zhou Z, Liu Y, Qin M, Sheng W, Wang X, Li Z, Zhong R (2014) Depletion of PKM2 leads to impaired glycolysis and cell death in 2-demethoxy-2,3-ethylenediamino hypocrellin B-photoinduced A549 cells. J Photochem Photobiol B 134:1–8. https://doi.org/10.1016/j.jphotobiol.2014.03.014
Ayyasamy V, Owens KM, Desouki MM, Liang P, Bakin A, Thangaraj K, Buchsbaum DJ, LoBuglio AF, Singh KK (2011) Cellular model of Warburg effect identifies tumor promoting function of UCP2 in breast cancer and its suppression by genipin. PLoS One 6(9):e24792. https://doi.org/10.1371/journal.pone.0024792
Bhattacharya B, Mohd Omar MF, Soong R (2016) The Warburg effect and drug resistance. Br J Pharmacol 173(6):970–979. https://doi.org/10.1111/bph.13422
Liberti MV, Locasale JW (2016) The Warburg effect: how does it benefit cancer cells? Trends Biochem Sci 41(3):211–218. https://doi.org/10.1016/j.tibs.2015.12.001
Suchorolski MT, Paulson TG, Sanchez CA, Hockenbery D, Reid BJ (2013) Warburg and Crabtree effects in premalignant Barrett’s esophagus cell lines with active mitochondria. PLoS One 8(2):e56884. https://doi.org/10.1371/journal.pone.0056884
Dong G, Mao Q, Xia W, Xu Y, Wang J, Xu L, Jiang F (2016) PKM2 and cancer: the function of PKM2 beyond glycolysis. Oncol Lett 11(3):1980–1986. https://doi.org/10.3892/ol.2016.4168
Zhang HS, Zhang FJ, Li H, Liu Y, Du GY, Huang YH (2016) Tanshinone A inhibits human esophageal cancer cell growth through miR-122-mediated PKM2 down-regulation. Arch Biochem Biophys 598:50–56. https://doi.org/10.1016/j.abb.2016.03.031
Golding JP, Wardhaugh T, Patrick L, Turner M, Phillips JB, Bruce JI, Kimani SG (2013) Targeting tumour energy metabolism potentiates the cytotoxicity of 5-aminolevulinic acid photodynamic therapy. Br J Cancer 109(4):976–982. https://doi.org/10.1038/bjc.2013.391
Agostinis P, Berg K, Cengel KA, Foster TH, Girotti AW, Gollnick SO, Hahn SM, Hamblin MR, Juzeniene A, Kessel D, Korbelik M, Moan J, Mroz P, Nowis D, Piette J, Wilson BC, Golab J (2011) Photodynamic therapy of cancer: an update. CA Cancer J Clin 61(4):250–281. https://doi.org/10.3322/caac.20114
Feng X, Zhang Y, Wang P, Liu Q, Wang X (2014) Energy metabolism targeted drugs synergize with photodynamic therapy to potentiate breast cancer cell death. Photochem Photobiol Sci 13(12):1793–1803. https://doi.org/10.1039/c4pp00288a
De Preter G, Neveu MA, Danhier P, Brisson L, Payen VL, Porporato PE, Jordan BF, Sonveaux P, Gallez B (2016) Inhibition of the pentose phosphate pathway by dichloroacetate unravels a missing link between aerobic glycolysis and cancer cell proliferation. Oncotarget 7(3):2910–2920. https://doi.org/10.18632/oncotarget.6272
Ju R, Guo L, Li J, Zhu L, Yu X, Chen C, Chen W, Ye C, Zhang D (2016) Carboxyamidotriazole inhibits oxidative phosphorylation in cancer cells and exerts synergistic anti-cancer effect with glycolysis inhibition. Cancer Lett 370(2):232–241. https://doi.org/10.1016/j.canlet.2015.10.025
Xintaropoulou C, Ward C, Wise A, Marston H, Turnbull A, Langdon SP (2015) A comparative analysis of inhibitors of the glycolysis pathway in breast and ovarian cancer cell line models. Oncotarget 6(28):25677–25695. https://doi.org/10.18632/oncotarget.4499
Ganapathy-Kanniappan S (2018) Molecular intricacies of aerobic glycolysis in cancer: current insights into the classic metabolic phenotype. Crit Rev Biochem Mol Biol 53(6):667–682. https://doi.org/10.1080/10409238.2018.1556578
Weijer R, Clavier S, Zaal EA, Pijls MM, van Kooten RT, Vermaas K, Leen R, Jongejan A, Moerland PD, van Kampen AH, van Kuilenburg AB, Berkers CR, Lemeer S, Heger M (2017) Multi-OMIC profiling of survival and metabolic signaling networks in cells subjected to photodynamic therapy. Cell Mol Life Sci 74(6):1133–1151. https://doi.org/10.1007/s00018-016-2401-0
van Niekerk G, Engelbrecht AM (2018) Role of PKM2 in directing the metabolic fate of glucose in cancer: a potential therapeutic target. Cell Oncol (Dordr) 41(4):343–351. https://doi.org/10.1007/s13402-018-0383-7
Wong N, Ojo D, Yan J, Tang D (2015) PKM2 contributes to cancer metabolism. Cancer Lett 356(2 Pt A):184–191. https://doi.org/10.1016/j.canlet.2014.01.031
Li S, Huang P, Gan J, Ling X, Du X, Liao Y, Li L, Meng Y, Li Y, Bai Y (2019) Dihydroartemisinin represses esophageal cancer glycolysis by down-regulating pyruvate kinase M2. Eur J Pharmacol 854:232–239. https://doi.org/10.1016/j.ejphar.2019.04.018
Parodi MB, Liberali T, Pedio M, Francis PJ, Piccolino FC, Fiotti N, Romano M, Ravalico G (2006) Photodynamic therapy of subfoveal choroidal neovascularization secondary to reticular pattern dystrophy: three-year results of an uncontrolled, prospective case series. Am J Ophthalmol 141(6):1152–1154. https://doi.org/10.1016/j.ajo.2006.01.038
Ji HT, Chien LT, Lin YH, Chien HF, Chen CT (2010) 5-ALA mediated photodynamic therapy induces autophagic cell death via AMP-activated protein kinase. Mol Cancer 9:91. https://doi.org/10.1186/1476-4598-9-91
Szokalska A, Makowski M, Nowis D, Wilczynski GM, Kujawa M, Wojcik C, Mlynarczuk-Bialy I, Salwa P, Bil J, Janowska S, Agostinis P, Verfaillie T, Bugajski M, Gietka J, Issat T, Glodkowska E, Mrowka P, Stoklosa T, Hamblin MR, Mroz P, Jakobisiak M, Golab J (2009) Proteasome inhibition potentiates antitumor effects of photodynamic therapy in mice through induction of endoplasmic reticulum stress and unfolded protein response. Cancer Res 69(10):4235–4243. https://doi.org/10.1158/0008-5472.CAN-08-3439
Ao R, Guan L, Wang Y, Wang JN (2017) Effects of PKM2 gene silencing on the proliferation and apoptosis of colorectal cancer LS-147T and SW620 cells. Cell Physiol Biochem 42(5):1769–1778. https://doi.org/10.1159/000479456
Azoitei N, Becher A, Steinestel K, Rouhi A, Diepold K, Genze F, Simmet T, Seufferlein T (2016) PKM2 promotes tumor angiogenesis by regulating HIF-1alpha through NF-kappaB activation. Mol Cancer 15:3. https://doi.org/10.1186/s12943-015-0490-2
Mukherjee J, Ohba S, See WL, Phillips JJ, Molinaro AM, Pieper RO (2016) PKM2 uses control of HuR localization to regulate p27 and cell cycle progression in human glioblastoma cells. Int J Cancer 139(1):99–111. https://doi.org/10.1002/ijc.30041
Liang J, Cao R, Wang X, Zhang Y, Wang P, Gao H, Li C, Yang F, Zeng R, Wei P, Li D, Li W, Yang W (2017) Mitochondrial PKM2 regulates oxidative stress-induced apoptosis by stabilizing Bcl2. Cell Res 27(3):329–351. https://doi.org/10.1038/cr.2016.159
Bhuvaneswari R, Thong PS, Gan YY, Soo KC, Olivo M (2010) Evaluation of hypericin-mediated photodynamic therapy in combination with angiogenesis inhibitor bevacizumab using in vivo fluorescence confocal endomicroscopy. J Biomed Opt 15(1):011114. https://doi.org/10.1117/1.3281671
Peng CL, Lin HC, Chiang WL, Shih YH, Chiang PF, Luo TY, Cheng CC, Shieh MJ (2018) Anti-angiogenic treatment (Bevacizumab) improves the responsiveness of photodynamic therapy in colorectal cancer. Photodiagn Photodyn Ther 23:111–118. https://doi.org/10.1016/j.pdpdt.2018.06.008
Hsu CC, Yang CS, Peng CH, Lee FL, Lee SM (2011) Combination photodynamic therapy and intravitreal bevacizumab used to treat circumscribed choroidal hemangioma. J Chin Med Assoc 74(10):473–477. https://doi.org/10.1016/j.jcma.2011.08.020
Bizjak M, Malavasic P, Dolinar K, Pohar J, Pirkmajer S, Pavlin M (2017) Combined treatment with Metformin and 2-deoxy glucose induces detachment of viable MDA-MB-231 breast cancer cells in vitro. Sci Rep 7(1):1761. https://doi.org/10.1038/s41598-017-01801-5
Acknowledgements
We are grateful to the Laboratory of Medical Genetics, Department of Biology, Harbin Medical University and State Key Laboratory of Molecular Oncology for their kind generosity of cells. This work was supported by the National Natural Science Foundation of China (81602662), the Wu Jieping Medical Foundation (320.6750.16002), and the Scientific Research Foundation of Harbin Medical University Cancer Hospital (BJQN2018-02).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gan, J., Li, S., Meng, Y. et al. The influence of photodynamic therapy on the Warburg effect in esophageal cancer cells. Lasers Med Sci 35, 1741–1750 (2020). https://doi.org/10.1007/s10103-020-02966-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-020-02966-8