Abstract
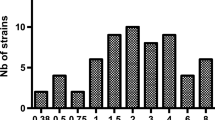
Pharmacological and clinical data regarding cefoxitin for the treatment of ESBL-producing Enterobacteriaceae-related infections are limited. We performed a multicentric prospective cohort study to evaluate continuous/prolonged, or intermittent infusion of cefoxitin. We assessed the plasma concentration as a function of the duration of infusion and then performed a simulation of the percentage of patients who would reach the PK/PD targets, set at 100% ƒT> MIC or 100% ƒT>4 MIC. Eighty-one patients were included. All patients were treated with 6 gr./day. MICs to cefoxitin ranged from 0.5 to 64 mg/L. Sixteen (19.7%) patients were infected with strains with cefoxitin MICs ≥ 8 mg/L. In all patients infected with strains with MICs ≤ 6 mg/L, PK/PD objectives (100% ƒT> MIC) were achieved with prolonged or continuous infusion. In contrast, when MICs were 8 mg/L only, continuous infusion was sufficient to achieve the PK/PD objectives (100% ƒT> MIC). Extended infusion of cefoxitin is necessary for the treatment of non-UTI ESBL-related infections.



Similar content being viewed by others
Abbreviations
- BSI:
-
bloodstream infection
- CI:
-
continuous infusion
- ESBL:
-
extended-spectrum beta-lactamase
- IB:
-
intermittent bolus
- MIC:
-
minimum inhibitory concentration
- PD:
-
pharmacodynamic
- PI:
-
prolonged infusion
- PK:
-
pharmacokinetic
- PTA:
-
probability of target attainment
- UTI:
-
urinary tract infusion
References
Jernigan JA, Hatfield KM, Wolford H, Nelson RE, Olubajo B, Reddy SC, et al. Multidrug-resistant bacterial infections in U.S. hospitalized patients, 2012-2017. N. Engl. J. Med. 2020;382:1309–19. doi: 10.1056/NEJMoa1914433
Barbier F, Pommier C, Essaied W, Garrouste-Orgeas M, Schwebel C, Ruckly S et al (2016) Colonization and infection with extended-spectrum β-lactamase-producing Enterobacteriaceae in ICU patients: what impact on outcomes and carbapenem exposure? J Antimicrob Chemother 71:1088–1097. https://doi.org/10.1093/jac/dkv423
Armand-Lefèvre L, Angebault C, Barbier F, Hamelet E, Defrance G, Ruppé E et al (2013) Emergence of imipenem-resistant gram-negative bacilli in intestinal flora of intensive care patients. Antimicrob Agents Chemother 57:1488–1495. https://doi.org/10.1128/AAC.01823-12
Bouxom H, Fournier D, Bouiller K, Hocquet D, Bertrand X (2018) Which non-carbapenem antibiotics are active against extended-spectrum β-lactamase-producing Enterobacteriaceae? Int. J Antimicrob Agents 52:100–103. https://doi.org/10.1016/j.ijantimicag.2018.03.014
Pilmis B, Parize P, Zahar JR, Lortholary O (2014) Alternatives to carbapenems for infections caused by ESBL-producing Enterobacteriaceae. Eur J Clin Microbiol Infect Dis Off Publ Eur Soc Clin Microbiol 33:1263–1265. https://doi.org/10.1007/s10096-014-2094-y
Craig WA (2003) Basic pharmacodynamics of antibacterials with clinical applications to the use of beta-lactams, glycopeptides, and linezolid. Infect Dis Clin N Am 17:479–501
De Waele J, Carlier M, Hoste E, Depuydt P, Decruyenaere J, Wallis SC et al (2014) Extended versus bolus infusion of meropenem and piperacillin: a pharmacokinetic analysis. Minerva Anestesiol 80:1302–1309
Roberts JA, Ulldemolins M, Roberts MS, McWhinney B, Ungerer J, Paterson DL et al (2010) Therapeutic drug monitoring of β-lactams in critically ill patients: proof of concept. Int J Antimicrob Agents 36:332–339. https://doi.org/10.1016/j.ijantimicag.2010.06.008
Roberts JA, Boots R, Rickard CM, Thomas P, Quinn J, Roberts DM, et al. Is continuous infusion ceftriaxone better than once-a-day dosing in intensive care? A randomized controlled pilot study. J. Antimicrob. Chemother. 2006 13;59:285–91. doi: 10.1093/jac/dkl478
Mambie A, Vuotto F, Poitrenaud D, Weyrich P, Cannesson O, Dessein R et al (2016) Cefoxitin: an alternative to carbapenems in urinary tract infections due to extended-spectrum beta-lactamase-producing Enterobacteriaceae. Med Mal Infect 46:215–219. https://doi.org/10.1016/j.medmal.2016.04.008
Demonchy E, Courjon J, Ughetto E, Durand M, Risso K, Garraffo R et al (2018) Cefoxitin-based antibiotic therapy for extended-spectrum β-lactamase-producing Enterobacteriaceae prostatitis: a prospective pilot study. Int J Antimicrob Agents 51:836–841. https://doi.org/10.1016/j.ijantimicag.2018.01.008
Isla A, Trocóniz IF, de Tejada IL, Vázquez S, Canut A, López JM et al (2012) Population pharmacokinetics of prophylactic cefoxitin in patients undergoing colorectal surgery. Eur J Clin Pharmacol 68:735–745. https://doi.org/10.1007/s00228-011-1206-1
EUCAST: clinical breakpoints [Internet]. [cited 2015 18];doi: http://www.eucast.org/clinical_breakpoints/
Schrogie JJ, Davies RO, Yeh KC, Rogers D, Holmes GI, Skeggs H et al (1978) Bioavailability and pharmacokinetics of cefoxitin sodium. J Antimicrob Chemother 4:69–78. https://doi.org/10.1093/jac/4.suppl_b.69
Senard O, Lafaurie M, Lesprit P, Nguyen Y, Lescure X, Therby A et al (2020) Efficacy of cefoxitin versus carbapenem in febrile male urinary tract infections caused by extended spectrum beta-lactamase-producing Escherichia coli: a multicenter retrospective cohort study with propensity score analysis. Eur J Clin Microbiol Infect Dis Off Publ Eur Soc Clin Microbiol 39:121–129. https://doi.org/10.1007/s10096-019-03701-0
Guet-Revillet H, Emirian A, Groh M, Nebbad-Lechani B, Weiss E, Join-Lambert O et al (2014) Pharmacological study of cefoxitin as an alternative antibiotic therapy to carbapenems in treatment of urinary tract infections due to extended-spectrum-β-lactamase-producing Escherichia coli. Antimicrob Agents Chemother 58:4899–4901. https://doi.org/10.1128/AAC.02509-14
Lodise TP, Lomaestro B, Drusano GL. Piperacillin-tazobactam for Pseudomonas aeruginosa infection: clinical implications of an extended-infusion dosing strategy. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2007 1;44:357–63. doi: 10.1086/510590
McKinnon PS, Paladino JA, Schentag JJ (2008) Evaluation of area under the inhibitory curve (AUIC) and time above the minimum inhibitory concentration (T>MIC) as predictors of outcome for cefepime and ceftazidime in serious bacterial infections. Int J Antimicrob Agents 31:345–351. https://doi.org/10.1016/j.ijantimicag.2007.12.009
Mouton JW, den Hollander JG (1994) Killing of Pseudomonas aeruginosa during continuous and intermittent infusion of ceftazidime in an in vitro pharmacokinetic model. Antimicrob Agents Chemother 38:931–936
Mouton JW, Vinks AA (2007) Continuous infusion of beta-lactams. Curr Opin Crit Care 13:598–606. https://doi.org/10.1097/MCC.0b013e3282e2a98f
Roosendaal R, Bakker-Woudenberg IA, van den Berghe-van Raffe M (1989) Vink-van den Berg JC, Michel BM. Impact of the dosage schedule on the efficacy of ceftazidime, gentamicin and ciprofloxacin in Klebsiella pneumoniae pneumonia and septicemia in leukopenic rats. Eur. J. Clin. Microbiol. Infect. Dis. Off. Publ. Eur. Soc. Clin. Microbiol. 8:878–887. https://doi.org/10.1007/bf01963774
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Pilmis, B., Mizrahi, A., Mory, C. et al. How to optimize administration of cefoxitin for the treatment of extended spectrum producing Enterobacteriaceae-related infection?. Eur J Clin Microbiol Infect Dis 40, 1393–1397 (2021). https://doi.org/10.1007/s10096-021-04165-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-021-04165-x