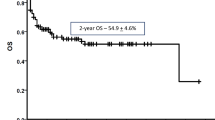
Abstract
A multicenter retrospective study in 131 patients (44 females/87 males) with hematological disorders who underwent G-CSF-primed/haplo-identical (Haplo-ID) (n = 76) or HLA-identical (HLA-ID) HSCT (n = 55) from February 2013 to February 2016 was conducted to compare the incidence and risk factors for pre-engraftment bloodstream infection (PE-BSI). In the Haplo-ID group, 71/76 patients with high-risk (n = 28) or relapsed/refractory hematological malignancies (n = 43) received FA5-BUCY conditioning (NCT02328950). All received trimethoprim–sulfamethoxazole (TMP–SMX) prophylaxis. Blood cultures and catheter tip cultures were obtained to confirm the BSI. PE-BSI was detected in 24/131 HSCT patients (18/76 in Haplo-ID and 6/55 in HLA-ID) after 28 febrile neutropenic episodes. Among 28 isolates for the 24 patients, 21 (75%) were Gneg bacteria, 6 (21.4%) Gpos and 1 (3.6%) fungi. Bacteria sources were central venous line infection (7/29.2%), gastroenteritis (6/25%), lower respiratory tract infection (LRTI; 5/20.8%), perianal skin infection (4/16.7%), and unknown (2/8.3%). The duration of neutropenia (P = 0.046) and previous Gneg bacteremia (P = 0.037) were important risk factors by univariate analysis, while the type of HSCT was not. A trend of TMP–SMX-resistant BSI in both groups may be due to routine antibacterial prophylaxis strategies. Our data show that G-CSF-primed Haplo-ID HSCT did not increase the risk of PE-BSI, even with intensive immunosuppressive treatments.

Similar content being viewed by others
References
Poutsiaka DD, Munson D, Price LL, Chan GW, Snydman DR (2011) Blood stream infection (BSI) and acute GVHD after hematopoietic SCT (HSCT) are associated. Bone Marrow Transplant 46:300–307
Blennow O, Mattsson J, Remberger M (2013) Pre-engraftment blood stream infection is a risk factor for acute GVHD grades II–IV. Bone Marrow Transplant 48(12):1583–1584
Mikulska M, Del Bono V, Raiola AM et al (2009) Blood stream infections in allogeneic hematopoietic stem cell transplant recipients: reemergence of Gram-negative rods and increasing antibiotic resistance. Biol Blood Marrow Transplant 15:47–53
Almyroudis NG, Fuller A, Jakubowski A, Sepkowitz K, Jaffe D, Small TN et al (2005) Pre- and post-engraftment bloodstream infection rates and associated mortality in allogeneic hematopoietic stem cell transplant recipients. Transpl Infect Dis 7:11–17
Gudiol C, Garcia-Vidal C, Arnan M, Sánchez-Ortega I, Patiño B, Duarte R, Carratalà J (2014) Etiology, clinical features and outcomes of pre-engraftment and post-engraftment bloodstream infection in hematopoietic SCT recipients. Bone Marrow Transplant 49(6):824–830
Hong J, Moon SM, Ahn HK, Sym SJ, Park YS, Park J, Cho YK, Cho EK, Shin DB, Lee JH (2013) Comparison of characteristics of bacterial bloodstream infection between adult patients with allogeneic and autologous hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 19(6):994–999
Meyer E, Beyersmann J, Bertz H et al (2007) Risk factor analysis of blood stream infection and pneumonia in neutropenic patients after peripheral blood stem-cell transplantation. Bone Marrow Transplant 39:173–178
Busca A, Cavecchia I, Locatelli F, D'Ardia S, De Rosa FG, Marmont F, Ciccone G, Baldi I, Serra R, Gaido E, Falda M (2012) Blood stream infections after allogeneic stem cell transplantation: a single-center experience with the use of levofloxacin prophylaxis. Transpl Infect Dis 14(1):40–48
Parody R, Martino R, Rovira R et al (2006) Severe infections after unrelated donor allogeneic hematopoietic stem cell transplantation in adults: comparison of cord blood transplantation with peripheral blood and bone marrow transplantation. Biol Blood Marrow Transplant 12:734–748
Blennow O, Ljungman P, Sparrelid E, Mattsson J, Remberger M (2014) Incidence, risk factors, and outcome of bloodstream infections during the pre-engraftment phase in 521 allogeneic hematopoietic stem cell transplantations. Transpl Infect Dis 16(1):106–114
Marena C, Zecca M, Carenini ML, Bruschi A, Bassi ML, Olivieri P, Azzaretti S, Locatelli F (2001) Incidence of, and risk factors for, nosocomial infections among hematopoietic stem cell transplantation recipients, with impact on procedure-related mortality. Infect Control Hosp Epidemiol 22(8):510–517
Junghanss C, Marr KA, Carter RA et al (2002) Incidence and outcome of bacterial and fungal infections following nonmyeloablative compared with myeloablative allogeneic hematopoietic stem cell transplantation: a matched control study. Biol Blood Marrow Transplant 8:512–520
Meunier F, Lukan C (2008) The first European Conference on Infections in Leukaemia - ECIL1: a current perspective. Eur J Cancer 44:2112–2117
Tomblyn M, Chiller T, Einsele H, Gress R, Sepkowitz K, Storek J, Wingard JR, Young JA, Boeckh MJ (2009) Guidelines for preventing infectious complications among hematopoietic cell transplantationrecipients: a global perspective. Biol Blood Marrow Transplant 15:1143–1238
Center for International Blood and Marrow Transplant Research (CIBMTR); National Marrow Donor Program (NMDP); European Blood and Marrow Transplant Group (EBMT); American Society of Blood and Marrow Transplantation (ASBMT); Canadian Blood and Marrow Transplant Group (CBMTG); Infectious Disease Society of America (IDSA); Society for Healthcare Epidemiology of America (SHEA); Association of Medical Microbiology and Infectious Diseases Canada (AMMI); Centers for Disease Control and Prevention (CDC) (2009) Guidelines for preventing infectious complications among hematopoietic cell transplant recipients: a global perspective. Bone Marrow Transplant 44(8):453–558
Yokoe D, Casper C, Dubberke E, Lee G, Muñoz P, Palmore T, Sepkowitz K, Young JA, Donnelly JP, Center for International Blood and Marrow Transplant Research; National Marrow Donor Program; European Blood and Marrow Transplant Group; American Society of Blood and Marrow Transplantation; Canadian Blood and Marrow Transplant Group; Infectious Disease Society of America; Society for Healthcare Epidemiology of America; Association of Medical Microbiology and Infectious Diseases Canada; Centers for Disease Control and Prevention (2009) Infection prevention and control in health-care facilities in which hematopoietic cell transplant recipients are treated. Bone Marrow Transplant 44(8):495–507
Xu LP, Chen H, Chen J et al (2018) The consensus on indications, conditioning regimen, and donor selection of allogeneic hematopoietic cell transplantation for hematological diseases in China-recommendations from the Chinese Society of Hematology. J Hematol Oncol 11(1):33
Network N C C. Genetic/familial high-risk assessment: breast and ovarian[J]. NCCN Clinical Practice
Cheson BD, Fisher RI, Barrington SF et al (2014) Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol 32(27):3059–3068
Tachibana T, Tanaka M, Takasaki H et al (2011) Pretransplant serum ferritin is associated with bloodstream infections within 100 days of allogeneic stem cell transplantation for myeloid malignancies. Int J Hematol 93(3):368–374
Kanda J, Mizumoto C, Ichinohe T et al (2011) Pretransplant serum ferritin and C-reactive protein as predictive factors for early bacterial infection after allogeneic hematopoietic cell transplantation. Bone Marrow Transplant 46:208–216
Wang Y, Liu Q-F, Xu L-P et al (2015) Haploidentical vs identical-sibling transplant for AML in remission: a multicenter, prospective study. Blood 125(25):3956–3962
Gratwohl A (2012) The EBMT risk score. Bone Marrow Transplant 47(6):749–756
Yang T, Lin Q, Ren J, Chen P, Yuan X, Luo X, Liu T, Zheng J, Zheng Z, Zheng X, Chen X, Zhang L, Zheng H, Chen Z, Hua X, Le S, Li J, Chen Z, Hu J (2016) A 5-day cytoreductive chemotherapy followed by haplo-identical hsct (FA5-BUCY) as a tumor-ablative regimen improved the survival of patients with advanced hematological malignancies. Oncotarget 7(48):78773–78786
Al-Zahrani H, Nassar A, Al-Mohareb F, Al-Sharif F, Mohamed S, Al-Anazi K, Patel M, Rasheed W, Saleh AJ, Bakr M, Ahmed S, Ibrahim K, Hussain F, Elkum N, Elhassan T, Nurgat Z, Chaudhri N, Aljurf M (2011) Fludarabine-based conditioning chemotherapy for allogeneic hematopoietic stem cell transplantation in acquired severe aplastic anemia. Biol Blood Marrow Transplant 17(5):717–722
Kim H, Lee JH, Joo YD, Bae SH, Hyun MS, Lee JH, Kim DY, Lee WS, Ryoo HM, Kim MK, Park JH, Lee KH, Cooperative Study Group A for Hematology (COSAH) (2012) A randomized comparison of cyclophosphamide vs. reduced dose cyclophosphamide plus fludarabine for allogeneic hematopoietic cell transplantation in patients with aplastic anemia and hypoplastic myelodysplastic syndrome. Ann Hematol 91(9):1459–1469
Marks R, Potthoff K, Hahn J, Ihorst G, Bertz H, Spyridonidis A, Holler E, Finke JM (2008) Reduced-toxicity conditioning with fludarabine, BCNU, and melphalan in allogeneic hematopoietic cell transplantation: particular activity against advanced hematologic malignancies. Blood 112(2):415–425
Glucksberg H, Storb R, Fefer A, Buckner CD, Neiman PE, Clift RA, Lerner KG, Thomas ED (1974) Clinical manifestations of graft-versus-host disease in human recipients of marrow from HL-A-matched sibling donors. Transplantation 18(4):295–304
Lee SJ, Vogelsang G, Flowers ME (2003) Chronic graft-versus-host disease. Biol Blood Marrow Transplant 9(4):215–233
Bader P, Beck J, Frey A et al (1998) Serial and quantitative analysis of mixed hematopoietic chimerism by PCR in patients with acute leukemias allows the prediction of relapse after allogeneic BMT. Bone Marrow Transplant 21(5):487–495
Horan T, Gaynes R (2004) Surveillance of nosocomial infections. In: Hospital epidemiology and infection control, 3rd edn. Philadelphia, Lippincott, Williams & Wilkeins, pp 1659–1702
DesJardin JA, Falagas ME, Ruthazer R, Griffith J, Wawrose D, Schenkein D et al (1999) Clinical utility of blood cultures drawn from indwelling central venous catheters in hospitalized patients with cancer. Ann Intern Med 131(9):641–647
Chinese Society of Hematology, Chinese Medical Association (2012) Chinese guidelines for the clinical application of antibacterial drugs for agranulocytosis with fever. Zhonghua Xue Ye Xue Za Zhi 33(8):693–696
Chinese Society of Hematology, Chinese Medical Association; Chinese Medical Doctor Association, Hematology Branch (2016) Chinese guidelines for the clinical application of antibacterial drugs for agranulocytosis with fever (2016). Zhonghua Xue Ye Xue Za Zhi 37(5):353–359
Averbuch D, Orasch C, Cordonnier C, Livermore DM, Mikulska M, Viscoli C, Gyssens IC, Kern WV, Klyasova G, Marchetti O, Engelhard D, Akova M, ECIL4, a joint venture of EBMT, EORTC, ICHS, ESGICH/ESCMID and ELN (2013) European guidelines for empirical antibacterial therapy for febrile neutropenic patients in the era of growing resistance: summary of the 2011 4th European conference on infections in leukemia. Haematologica 98(12):1826–1835
Averbuch D, Cordonnier C, Livermore DM, Mikulska M, Orasch C, Viscoli C, Gyssens IC, Kern WV, Klyasova G, Marchetti O, Engelhard D, Akova M, ECIL4, a joint venture of EBMT, EORTC, ICHS, ESGICH/ESCMID and ELN (2013) Targeted therapy against multi-resistant bacteria in leukemic and hematopoietic stem cell transplant recipients: guidelines of the 4th European Conference on Infections in Leukemia (ECIL-4, 2011). Haematologica 98(12):1836–1847
Poutsiaka DD, Price LL, Ucuzian A, Chan GW, Miller KB, Snydman DR (2007) Blood stream infection after hematopoietic stem cell transplantation is associated with increased mortality. Bone Marrow Transplant 40(1):63–70
Huang XJ, Liu DH, Liu KY et al (2006) Haploidentical hematopoietic stem cell transplantation without in vitro T cell depletion for the treatment of hematological malignancies. Bone Marrow Transplant 38:291–297
Tang W, Fan X, Wang L, Hu J (2015) Busulfan and fludarabine conditioning regimen given at hematological nadir of cytoreduction fludarabine, cytarabine, and idarubicin chemotherapy in patients with refractory acute myeloid leukemia undergoing allogeneic stem cell transplantation: a single arm pilot consort study. Medicine 94(15):e706
Johansson JE, Brune M, Ekman T. (2001) The gut mucosa barrier is preserved during allogeneic, haemopoietic stem cell transplantation with reduced intensity conditioning[J]. Bone marrow transplantation 28(8):737
Niscola P, Romani C, Cupelli L et al (2007) Mucositis in patients with hematologic malignancies: an overview. Haematologica 92(2):222–231
Oliveira AL, de Souza M, Carvalho-Dias VM et al (2007) Epidemiology of bacteremia and factors associated with multi-drug-resistant gram-negative bacteremia in hematopoietic stem cell transplant recipients. Bone Marrow Transplant 39:775–781
Mikulska M, Del Bono V, Raiola AM, Bruno B, Gualandi F, Occhini D et al (2009) Bloodstream infections in allo-geneic hematopoietic stem cell transplant recipients: re-emergence of gram-negative rods and increasing antibiotic resistance. Biol Blood Marrow Transplant 15:47–53
Therriault BL, Wilson JW, Barreto JN, Estes LL (2010) Characterization of bacterial infection in allogenic hematopoietic stem cell transplant recipients who received prophylactic levofloxacin with either penicillin or doxacycline. Mayo Clin Proc 85(8):711–816
Liu CY, Lai YC, Huang LJ, Yang YW, Chen TL, Hsiao LT et al (2011) Impact of bloodstream infections on outcome and the influence of prophylactic oral antibiotic regimens in allogeneic hematopoietic SCT recipients. Bone Marrow Transplant 46:1231–1239
El-Mahallawy H, Samir I, Abdel Fattah R, Kadry D, El-Kholy A (2014) Source, pattern and antibiotic resistance of blood stream infections in hematopoietic stem cell transplant recipients. J Egypt Natl Canc Inst 26(2):73–77
Acknowledgments
This work was supported in part by the National and Fujian Provincial Key Clinical Specialty Discipline Construction Program, China; National High Technology Research and Development Program of China; 863 program (2012AA02A505); National Public Health Grand Research Foundation (201202017); National Natural Science Foundation of China (81570162, 81700131, 81870138); Fujian Provincial Key Laboratory Foundation of Hematology (2009J1004); and Natural Science Foundation of Fujian Province (2017Y9017, 2017Y9037, 2017Y9056, 2018Y0031). The authors would like to thank all the nurses and physicians at the center who all contributed to the management of patient care, and Dr. Martine Torres for her critical reading of the manuscript and editorial assistance.
Author information
Authors and Affiliations
Contributions
Prof. Jianda Hu and Prof. Ting Yang designed and performed the research. Dr. Jinhua Ren and Dr. Qiaoxian Ling collected the data. Dr. Qiaoxian Ling performed the statistical analyses. Prof. Ting Yang interpreted the data and wrote the manuscript. All authors participated in the management of patient care. All authors critically reviewed and approved the manuscript.
Corresponding author
Ethics declarations
This study was conducted in accordance with the ethical standards of the local institutional review board (the Medical Ethics Committee of Fujian Medical University Union Hospital and the Helsinki Declaration). Informed consent was obtained from all patients and donors before they were included in the study.
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ren, J., Lin, Q., Chen, W. et al. G-CSF-primed haplo-identical HSCT with intensive immunosuppressive and myelosuppressive treatments does not increase the risk of pre-engraftment bloodstream infection: a multicenter case–control study. Eur J Clin Microbiol Infect Dis 38, 865–876 (2019). https://doi.org/10.1007/s10096-019-03482-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-019-03482-6