Abstract
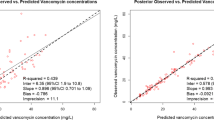
Vancomycin pharmacokinetic (PK) and pharmacodynamic (PD) data in neonates are based on total concentrations. However, only unbound vancomycin is pharmacologically active. The objective was to determine vancomycin protein binding and the covariates impacting unbound vancomycin concentration in neonates and young infants. In neonates and young infants to whom vancomycin was administered intermittently for medical indications, total and unbound vancomycin plasma concentrations were determined using LC-MS/MS. Sampling occurred randomly during vancomycin exposure, covering a broad range of concentrations. Impact of covariates on unbound vancomycin concentration was determined using linear regression. Significant results of the univariate regressions were entered in a stepwise multiple regression. Passing-Bablok regression and Bland-Altman were used to assess the difference between measured and calculated unbound vancomycin concentration. Thirty-seven samples in 33 patients (median (interquartile range) gestational age 35 (29–39) weeks) were collected. Median total and unbound vancomycin concentrations were 14.2 (7.4–20.6) and 13.6 (7.2–22.5) mg/L, respectively. Median unbound fraction was 0.90 (0.77–0.98). Multiple regression revealed total vancomycin concentration (β = 0.884, p < 0.001) and albumin (β = − 0.323, p = 0.007) as most important covariates of unbound vancomycin concentrations, with an R2 adjusted of 0.953 (p < 0.0001). Mean absolute difference between calculated and measured unbound vancomycin was − 0.008 (95% CI − 0.92–0.91) mg/L. The unbound vancomycin fraction in neonates is higher compared to that in children and adults, and total vancomycin concentration and albumin were the most important covariates of unbound vancomycin concentration. Integration of protein binding in future PK/PD analyses is appropriate to optimize vancomycin dosing and to determine population-specific vancomycin PD targets for neonates.


Similar content being viewed by others
References
Li S, Starkey ES (2016) What do I need to know about glycopeptide antibiotics? Arch Dis Child Educ Pract Ed 101:323–326
de Hoog M, Mouton JW, van den Anker JN (2004) Vancomycin: pharmacokinetics and administration regimens in neonates. Clin Pharmacokinet 43:417–440
Pacifici GM, Allegaert K (2012) Clinical pharmacokinetics of vancomycin in the neonate: a review. Clinics (Sao Paulo) 67:831–837
Vandendriessche A, Allegaert K, Cossey V, Naulaers G, Saegeman V, Smits A (2014) Prospective validation of neonatal vancomycin dosing regimens is urgently needed. Curr Ther Res Clin Exp 76:51–57
Bhongsatiern J, Stockmann C, Roberts JK, Yu T, Korgenski KE, Spigarelli MG, Desai PB, Sherwin CM (2015) Evaluation of vancomycin use in late-onset neonatal sepsis using the area under the concentration-time curve to the minimum inhibitory concentration >/=400 target. Ther Drug Monit 37:756–765
Petrie K, O'Brien C, Bhushan S, Tonna A (2015) Neonatal vancomycin trough level audit using British National Formulary for Children dosing. Arch Dis Child Fetal Neonatal Ed 100:F278–F279
Jacqz-Aigrain E, Leroux S, Zhao W, van den Anker JN, Sharland M (2015) How to use vancomycin optimally in neonates: remaining questions. Expert Rev Clin Pharmacol 8:635–648
Sammons HM, Starkey E (2013) Vancomycin use in neonates and children: evidence-based practice is needed. Arch Dis Child 98:447–448
Frymoyer A, Lee S, Bonifacio SL, Meng L, Lucas SS, Guglielmo BJ, Sun Y, Verotta D (2013) Every 36-h gentamicin dosing in neonates with hypoxic-ischemic encephalopathy receiving hypothermia. J Perinatol 33:778–782
Oyaert M, Spriet I, Allegaert K, Smits A, Vanstraelen K, Peersman N, Wauters J, Verhaegen J, Vermeersch P, Pauwels S (2015) Factors impacting unbound vancomycin concentrations in different patient populations. Antimicrob Agents Chemother 59:7073–7079
Anderson BJ, Allegaert K, van den Anker JN, Cossey V, Holford NH (2007) Vancomycin pharmacokinetics in preterm neonates and the prediction of adult clearance. Br J Clin Pharmacol 63:75–84
Ackerman BH, Taylor EH, Olsen KM, Abdel-Malak W, Pappas AA (1988) Vancomycin serum protein binding determination by ultrafiltration. Drug Intell Clin Pharm 22:300–303
Butterfield JM, Patel N, Pai MP, Rosano TG, Drusano GL, Lodise TP (2011) Refining vancomycin protein binding estimates: identification of clinical factors that influence protein binding. Antimicrob Agents Chemother 55:4277–4282
Oyaert M, Peersman N, Kieffer D, Deiteren K, Smits A, Allegaert K, Spriet I, Van Eldere J, Verhaegen J, Vermeersch P, Pauwels S (2015) Novel LC-MS/MS method for plasma vancomycin: comparison with immunoassays and clinical impact. Clin Chim Acta 441:63–70
Stove V, Coene L, Carlier M, De Waele JJ, Fiers T, Verstraete AG (2015) Measuring unbound versus total vancomycin concentrations in serum and plasma: methodological issues and relevance. Ther Drug Monit 37:180–187
De Cock PA, Desmet S, De Jaeger A, Biarent D, Dhont E, Herck I, Vens D, Colman S, Stove V, Commeyne S, Vande Walle J, De Paepe P (2017) Impact of vancomycin protein binding on target attainment in critically ill children: back to the drawing board? J Antimicrob Chemother 72(3):801–804
Crandon JL, MacVane SH, Nicolau DP (2013) Clinical laboratory-based assay methodologies may underestimate and increase variability of vancomycin protein binding in hospitalized patients. Pharmacotherapy 34(2):203–209
Kees MG, Wicha SG, Seefeld A, Kees F, Kloft C (2014) Unbound fraction of vancomycin in intensive care unit patients. J Clin Pharmacol 54:318–323
Berthoin K, Ampe E, Tulkens PM, Carryn S (2009) Correlation between free and total vancomycin serum concentrations in patients treated for Gram-positive infections. Int J Antimicrob Agents 34:555–560
Ampe E, Delaere B, Hecq JD, Tulkens PM, Glupczynski Y (2013) Implementation of a protocol for administration of vancomycin by continuous infusion: pharmacokinetic, pharmacodynamic and toxicological aspects. Int J Antimicrob Agents 41:439–446
Rodvold KA, Blum RA, Fischer JH, Zokufa HZ, Rotschafer JC, Crossley KB, Riff LF (1988) Vancomycin pharmacokinetics in patients with various degrees of renal function. Antimicrob Agents Chemother 32:848–852
Tan CC, Lee HS, Ti TY, Lee EJ (1990) Pharmacokinetics of intravenous vancomycin in patients with end-stage renal failure. Ther Drug Monit 12:29–34
Zokufa HZ, Solem LD, Rodvold KA, Crossley KB, Fischer JH, Rotschafer JC (1989) The influence of serum albumin and alpha 1-acid glycoprotein on vancomycin protein binding in patients with burn injuries. J Burn Care Rehabil 10:425–428
Sando M, Sato Y, Iwata S, Akita H, Sunakawa K (2004) In vitro protein binding of teicoplanin to neonatal serum. J Infect Chemother 10:280–283
Smits A, Kulo A, Verbesselt R, Naulaers G, de Hoon J, Vermeersch P, Allegaert K (2012) Cefazolin plasma protein binding and its covariates in neonates. Eur J Clin Microbiol Infect Dis 31:3359–3365
Pullen J, Stolk LM, Degraeuwe PL, van Tiel FH, Neef C, Zimmermann LJ (2007) Protein binding of flucloxacillin in neonates. Ther Drug Monit 29:279–283
Schmidt S, Gonzalez D, Derendorf H (2010) Significance of protein binding in pharmacokinetics and pharmacodynamics. J Pharm Sci 99:1107–1122
Roberts JA, Pea F, Lipman J (2012) The clinical relevance of plasma protein binding changes. Clin Pharmacokinet 52:1–8
Zeitlinger MA, Derendorf H, Mouton JW, Cars O, Craig WA, Andes D, Theuretzbacher U (2011) Protein binding: do we ever learn? Antimicrob Agents Chemother 55:3067–3074
Sinkeler FS, de Haan TR, Hodiamont CJ, Bijleveld YA, Pajkrt D, Mathot RA (2014) Inadequate vancomycin therapy in term and preterm neonates: a retrospective analysis of trough serum concentrations in relation to minimal inhibitory concentrations. BMC Pediatr 14:193
Antimicrobial wild type distribution of microorganisms. European Committee on Antimicrobial Susceptibility Testing. Data from the EUCAST MIC distribution website, last accessed 21–01-2018. http://mic.eucast.org/Eucast2/
Padari H, Oselin K, Tasa T, Metsvaht T, Loivukene K, Lutsar I (2016) Coagulase negative staphylococcal sepsis in neonates: do we need to adapt vancomycin dose or target? BMC Pediatr 16:206
European Committee on Antimicrobial Susceptibility Testing. Data from the EUCAST website, Rationale for the EUCAST vancomycin clinical breakpoints, last accessed 21–01-2018 http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Rationale_documents/Vancomycin_rationale_2.1
Ulldemolins M, Roberts JA, Rello J, Paterson DL, Lipman J (2011) The effects of hypoalbuminaemia on optimizing antibacterial dosing in critically ill patients. Clin Pharmacokinet 50:99–110
Smits A, Roberts JA, Vella-Brincat JWA, Allegaert K (2014) Cefazolin plasma protein binding in different human populations: more than cefazolin-albumin interaction. Int J Antimicrob Agents 43:195–200
Notarianni LJ (1990) Plasma protein binding of drugs in pregnancy and in neonates. Clin Pharmacokinet 18:20–36
Stoop JW, Zegers BJ, Sander PC, Ballieux RE (1969) Serum immunoglobulin levels in healthy children and adults. Clin Exp Immunol 4:101–112
Alkan OS, Ozer EA, Kose S, Ilhan O, Ozturk C, Sutcuoglu S (2016) Reference values of serum IgG and IgM levels in preterm and term newborns. J Matern Fetal Neonatal Med 29:972–976
Ballow M, Cates KL, Rowe JC, Goetz C, Desbonnet C (1986) Development of the immune system in very low birth weight (less than 1500 g) premature infants: concentrations of plasma immunoglobulins and patterns of infections. Pediatr Res 20:899–904
Stoll BJ, Hansen N, Fanaroff AA, Wright LL, Carlo WA, Ehrenkranz RA, Lemons JA, Donovan EF, Stark AR, Tyson JE, Oh W, Bauer CR, Korones SB, Shankaran S, Laptook AR, Stevenson DK, Papile LA, Poole WK (2002) Late-onset sepsis in very low birth weight neonates: the experience of the NICHD Neonatal Research Network. Pediatrics 110:285–291
Funding
K. Allegaert has been supported by the Fund for Scientific Research, Flanders (Belgium) (FWO Vlaanderen), by a fundamental Clinical Investigatorship (1800214N). The research activities of A. Smits, S. Pauwels, and I. Spriet were supported by the Clinical Research and Education Council of the University Hospitals Leuven. The research was further facilitated by the “Agency for Innovation, Science and Technology in Flanders” (IWT) through the “SAFEDRUG” project (IWT/SBO 130033).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed parental consent was obtained for all included participants.
Data availability statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Rights and permissions
About this article
Cite this article
Smits, A., Pauwels, S., Oyaert, M. et al. Factors impacting unbound vancomycin concentrations in neonates and young infants. Eur J Clin Microbiol Infect Dis 37, 1503–1510 (2018). https://doi.org/10.1007/s10096-018-3277-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-018-3277-8