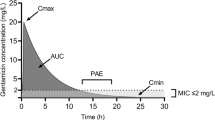
Abstract
Previous studies have shown that the high dose of gentamicin (8 mg/kg) rarely achieves the desired peak plasma concentration (Cmax) of ≥30 mg/l in patients with severe sepsis or septic shock. The aim of this study was to determine the first dose of gentamicin needed to achieve a Cmax ≥ 30 mg/l. We conducted a prospective observational cohort study in one intensive care unit. All consecutive patients hospitalized for severe sepsis or septic shock and treated with a first dose of gentamicin >6 mg/kg were evaluated. During the study period, 15 of the 57 patients (26.3 %) treated with gentamicin had a Cmax ≥ 30 mg/l. The median dose of gentamicin administered was 8.9 [7.8–9.9] mg/kg. Independent factors in the multivariate analysis associated with a Cmax ≥ 30 mg/l were higher body mass index (per kg/m2 increment) (OR: 1.173, 95%CI: 1.015–1.356, P = 0.03) and higher first dose of gentamicin (per mg/kg increment) (OR: 2.343, 95%CI: 1.346–4.08, P = 0.003). The optimal first dose to achieve a Cmax ≥ 30 mg/l was 11 mg/kg, with a specificity and a sensitivity of 100 % and 53.3 % respectively. These results suggest that a first dose of gentamicin >11 mg/kg is needed to achieve a Cmax ≥ 30 mg/l in most patients.

Similar content being viewed by others
Abbreviations
- Cmax:
-
Peak plasma concentration
- ICU:
-
Intensive care unit
- IBW:
-
Ideal body weight
- EUCAST:
-
European Committee on Antimicrobial Susceptibility Testing
- MIC:
-
Minimal inhibiting concentration
- SAPS:
-
Simplified acute physiology score
- SOFA:
-
Sequential organ failure assessment
References
Agence française de sécurité sanitaire des produits de santé (2012) Update on good use of injectable aminoglycosides, gentamycin, tobramycin, netilmycin, amikacin. Pharmacological properties, indications, dosage, and mode of administration, treatment monitoring. Med Mal Infect 42:301–308
Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, Sevransky JE, Sprung CL, Douglas IS, Jaeschke R, Osborn TM, Nunnally ME, Townsend SR, Reinhart K, Kleinpell RM, Angus DC, Deutschman CS, Machado FR, Rubenfeld GD, Webb S, Beale RJ, Vincent JL, Moreno R, Surviving Sepsis Campaign Guidelines Committee including The Pediatric Subgroup (2013) Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med 39:165–228
Moore RD, Lietman PS, Smith CR (1987) Clinical response to aminoglycoside therapy: importance of the ratio of peak concentration to minimal inhibitory concentration. J Infect Dis 155:93–99
EUCAST. European Committee on Antimicrobial Susceptibility Testing. www.eucast.org/. 2015
Roger C, Nucci B, Louart B, Friggeri A, Knani H, Evrard A, Lavigne JP, Allaouchiche B, Lefrant JY, Roberts JA, Muller L (2016) Impact of 30 mg/kg amikacin and 8 mg/kg gentamicin on serum concentrations in critically ill patients with severe sepsis. J Antimicrob Chemother 71:208–212
Allou N, Allyn J, Levy Y, Bouteau A, Caujolle M, Delmas B, Valance D, Brulliard C, Martinet O, Vandroux D, Montravers P, Augustin P (2016) Assessment of the National French recommendations regarding the dosing regimen of 8 mg/kg of gentamicin in patients hospitalized in intensive care unit. Anaesth Crit Care Pain Med [In press]
von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP (2007) The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet 370:1453–1457
Blackburn LM, Tverdek FP, Hernandez M, Bruno JJ (2015) First-dose pharmacokinetics of aminoglycosides in critically ill haematological malignancy patients. Int J Antimicrob Agents 45:46–53
Duszynska W, Taccone FS, Hurkacz M, Kowalska-Krochmal B, Wiela-Hojenska A, Kubler A (2013) Therapeutic drug monitoring of amikacin in septic patients. Crit Care 17:R165
Traynor AM, Nafziger AN, Bertino JS Jr (1995) Aminoglycoside dosing weight correction factors for patients of various body sizes. Antimicrob Agents Chemother 39:545–548
Roger C, Nucci B, Molinari N, Bastide S, Saissi G, Pradel G, Barbar S, Aubert C, Lloret S, Elotmani L, Polge A, Lefrant JY, Roberts JA, Muller L (2015) Standard dosing of amikacin and gentamicin in critically ill patients results in variable and subtherapeutic concentrations. Int J Antimicrob Agents 46:21–27
Montravers P, Dufour G, Guglielminotti J, Desmard M, Muller C, Houissa H, Allou N, Marmuse JP, Augustin P (2015) Dynamic changes of microbial flora and therapeutic consequences in persistent peritonitis. Crit Care 2(19):70
Bertino JS Jr, Booker LA, Franck PA, Jenkins PL, Franck KR, Nafziger AN (1993) Incidence of and significant risk factors for aminoglycoside-associated nephrotoxicity in patients dosed by using individualized pharmacokinetic monitoring. J Infect Dis 167:173–179
Maller R, Ahrne H, Holmen C, Lausen I, Nilsson LE, Smedjegard J (1993) Once- versus twice-daily amikacin regimen: efficacy and safety in systemic gram-negative infections. Scandinavian Amikacin Once Daily Study Group. J Antimicrob Chemother 31:939–948
Oliveira JF, Silva CA, Barbieri CD, Oliveira GM, Zanetta DM, Burdmann EA (2009) Prevalence and risk factors for aminoglycoside nephrotoxicity in intensive care units. Antimicrob Agents Chemother 53:2887–2891
Taccone FS, Laterre PF, Spapen H, Dugernier T, Delattre I, Layeux B, De Backer D, Wittebole X, Wallemacq P, Vincent JL, Jacobs F (2010) Revisiting the loading dose of amikacin for patients with severe sepsis and septic shock. Crit Care 14:R53
Schneider AG, Thorpe C, Dellbridge K, Matalanis G, Bellomo R (2013) Electronic bed weighing vs daily fluid balance changes after cardiac surgery. J Crit Care 28(1113):e1–e5
Acknowledgments
Author Contributions
Drs Allou and Charifou had full access to all of the data in the study, and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design
Allou, Allyn, Galas, Valance, Martinet, and Vandroux.
Acquisition of data
Martinet, Valance, and Charifou.
Analysis and interpretation of data
Allou, Allyn, Martinet, Vandroux, and Valance.
Drafting of the manuscript
Allou, Galas, Martinet, Valance, and Allyn.
Critical revision of the manuscript for important intellectual content
Allou, Allyn, Vandroux, and Valance.
Statistical analysis
Allou, Allyn.
Obtained funding
Support was provided solely from institutional and/or departmental sources.
Administrative, technical, or material support
Allou, Allyn, Martinet, Charifou, and Galas.
Study supervision
Allou, Allyn, Charifou, and Valance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This work was internally funded
Conflict of Interest
The authors declare that they have no conflict of interest
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards (This observational study was approved by the Ethics Committee of Félix Guyon University Hospital (R15015))
Informed consent
Informed consent was obtained from all individual participants or their legally authorized representative included in the study.
Rights and permissions
About this article
Cite this article
Allou, N., Charifou, Y., Augustin, P. et al. A study to evaluate the first dose of gentamicin needed to achieve a peak plasma concentration of 30 mg/l in patients hospitalized for severe sepsis. Eur J Clin Microbiol Infect Dis 35, 1187–1193 (2016). https://doi.org/10.1007/s10096-016-2652-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-016-2652-6