Abstract
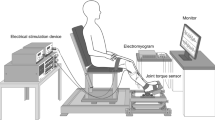
Motor imagery (MI) is known to improve motor function through enhancement of motor cortex activity. Spinal reciprocal inhibition (RI) is modulated by motor cortex activity, and, therefore, MI may change RI. The aim of this study was to examine the changes in RI during MI involving the lower extremity. Spinal RI was measured from the tibialis anterior (TA) to the soleus (SOL). Eleven healthy adults participated in experiment 1. All participants performed the following three conditions, and RI was assessed during each condition: (1) resting condition; (2) MI of ankle dorsiflexion condition (MI-DF); and (3) MI of ankle plantarflexion condition (MI-PF). Twelve healthy adults participated in experiment 2. All participants performed the following two conditions, and RI was assessed before and after MI practice for 10 min: (1) resting condition and (2) MI-DF. The interval between the conditioning and test stimulus (inter-stimulus interval; ISI) was set at 0, 1, 2, or 3 ms and 20 ms. In experiment 1, RI during MI-PF was significantly decreased compared with that during resting with both stimulus intervals. RI during MI-DF showed no significant change compared with that during resting with both ISIs. In experiment 2, the difference between the rest condition and the MI-DF condition after the MI task with ISI of 20 ms was significantly higher than before the MI task. Our findings suggest that real-time changes in RI during MI involving the lower extremity may vary depending on the direction of motion and MI practice.





Similar content being viewed by others
References
Decety J, Grèzes J (1999) Neural mechanisms subserving the perception of human actions. Trends Cogn Sci 3:172–178
Ehrsson HH, Geyer S, Naito E (2003) Imagery of voluntary movement of fingers, toes and tongue activates corresponding body-part specific motor representations. J Neurophysiol 90:3304–3316
Chen X, Wan L, Qin W, Zheng W, Qi Z, Chen N, Li K (2016) Functional preservation and reorganization of brain during motor imagery in patients with incomplete spinal cord injury: a pilot fMRI study. Front Hum Neurosci 10:46. https://doi.org/10.3389/fnhum.2016.00046
Bakker M, Overeem S, Snijders AH, Borm G, van Elwijik G, Toni I et al (2008) Motor imagery of foot dorsiflexion and gait: effects on corticospinal excitability. Clin Neurophysiol 119:2519–2527. https://doi.org/10.1016/j.clinph.2008.07.282
Lebon F, Byblow WD, Collet C, Guillot A, Stinear CM (2012) The modulation of motor cortex excitability during motor imagery depends on imagery quality. Eur J Neurosci 35:323–331. https://doi.org/10.1111/j.1460-9568.2011.07938.x
Kato K, Kanosue K (2017) Corticospinal excitability for hand muscles during motor imagery of foot changes with imagined force level. PLoS One 12:e0185547. https://doi.org/10.1371/journal.pone.0185547
García Carrasco D, Aboitiz Cantalapiedr J (2016) Effectiveness of motor imagery or mental practice in functional recovery after stroke: a systematic review. Neurología 31(1):43–52
Kho AY, Liu KPY, Chung RCK (2014) Meta-analysis on the effect of mental imagery on motor recovery of the hemiplegic upper extremity function. Aust Occup Ther J 61(2):38–48
Bonnet M, Decety J, Jeannerod M, Requin J (1997) Mental simulation of an action modulates the excitability of spinal reflex pathways in man. Cogn Brain Res 5(3):221–228
Aoyama T, Kaneko F (2011) The effect of motor imagery on gain modulation of the spinal reflex. Brain Res 1372:41–48. https://doi.org/10.1016/j.brainres.2010.11.023
Aymard C, Chia L, Katz R, Lafitte C, Pénicaud A (1995) Reciprocal inhibition between wrist flexors and extensors in man: a new set of interneurons ? J Physiol 487(1):221–235
Lundberg A (1970) The excitatory control of the Ia inhibitory pathway. In: Anderson P, Jensen JKS (eds) Excitatory synaptic mechanisms. The University Press, Oslo, Norway, pp 333–340
Morita H, Crone C, Christenhuis D, Petersen NT, Nielsen JB (2001) Modulation of presynaptic inhibition and disynaptic reciprocal Ia inhibition during voluntary movement in spasticity. Brain 124(Pt 4):826–837
Okuma Y, Mizuno Y, Lee RG (2002) Reciprocal Ia inhibition in patients with asymmetric spinal spasticity. Clin Neurophysiol 113:292–297. https://doi.org/10.1016/S1388-2457(02)00004-4
Crone C, Johnsen LL, Biering-Sørensen F, Nielsen B (2003) Appearance of reciprocal facilitation of ankle extensors from ankle flexors in patients with stroke or spinal cord injury. Brain. 126:495–507. https://doi.org/10.1093/brain/awg036
Knikou M, Mummidisetty CK (2011) Reduced reciprocal inhibition during assisted stepping in human spinal cord injury. Exp Neurol 231(1):104–112. https://doi.org/10.1016/j.expneurol.2011.05.021
Bhagchandani N, Schindler-Ivens S (2012) Reciprocal inhibition post-stroke is related to reflex excitability and movement ability. Clin Neurophysiol 123(11):2239–2246. https://doi.org/10.1016/j.clinph.2012.04.023
Wolpaw JR, Tennissen AM (2001) Activity-dependent spinal cord plasticity in health and disease. Annu Rev Neurosci 24:807–843
Chen XY, Chen L, Chen Y, Wolpaw JR (2006) Operant conditioning of reciprocal inhibition in rat soleus muscle. J Neurophysiol 96:2144–2150
Fujiwara T, Tsuji T, Honaga K, Hase K, Ushiba J, Liu M (2011 Sep) Transcranial direct current stimulation modulates the spinal plasticity induced with patterned electrical stimulation. Clin Neurophysiol 122(9):1834–1837. https://doi.org/10.1016/j.clinph.2011.02.002
Yamaguchi T, Fujiwara T, Saito K, Tanabe S, Muraoka Y, Otaka Y, Osu R, Tsuji T, Hase K, Liu M (2013 Feb) The effect of active pedaling combined with electrical stimulation on spinal reciprocal inhibition. J Electromyogr Kinesiol 23(1):190–194. https://doi.org/10.1016/j.jelekin.2012.08.007
Yamaguchi T, Fujiwara T, Tsai YA, Tang SC, Kawakami M, Mizuno K, Kodama M, Masakado Y, Liu M (2016) The effects of anodal transcranial direct current stimulation and patterned electrical stimulation on spinal inhibitory interneurons and motor function in patients with spinal cord injury. Exp Brain Res 234(6):1469–1478
Takahashi Y, Fujiwara T, Yamaguchi T, Kawakami M, Mizuno K, Liu M (2017) The effects of patterned electrical stimulation combined with voluntary contraction on spinal reciprocal inhibition in healthy individuals. Neuroreport. 28(8):434–438
Kawakami M, Okuyama K, Takahashi Y, Hiramoto M, Nishimura A, Ushiba J, Fujiwara T, Liu M (2018) Change in reciprocal inhibition of the forearm with motor imagery among patients with chronic stroke. Neural Plast 3946367:1–9. https://doi.org/10.1155/2018/3946367
Crone C, Hultborn H, Mazieres L, Morin C, Nielsen J, Pierrot Deseilligny E (1990) Sensitivity of monosynaptic test reflex to facilitation and inhibition as a function of the test reflex size: a study in man and the cat. Exp Brain Res 81:35–45. https://doi.org/10.1007/BF00230098
Mizuno Y, Tanaka R, Yanagisawa N (1971) Reciprocal group I inhibition on triceps surae motoneurons in man. J Neurophysiol 34:1010–1017
Tanaka R (1974) Reciprocal Ia inhibition during voluntary movements in man. Exp Brain Res 21:529–540. https://doi.org/10.1007/BF00237171
Robert R, Allow N, Hardy L, Markland D, Bringer J (2008) Movement imagery ability: development and assessment of a revised version of the vividness of movement imagery questionnaire. J Sport Exerc Psychol 30:200–221. https://doi.org/10.1123/jsep.30.2.200
Ikai T, Findley TW, Izumi S et al (1996) Reciprocal inhibition in the forearm during voluntary contraction and thinking about movement. Electroencephalogr Clin Neurophysiol 36(5):295–304
Crone C, Hultborn H, Jespersen B, Nielsen J (1987) Reciprocal Ia inhibition between ankle flexors and extensors in man. J Physiol 389:163–185
Trinastic JP, Kautz SA, McGregor K, Gregory C, Bowden M, Benjamin MB et al (2010) An fMRI study of the differences in brain activity during active ankle dorsiflexion and plantarflexion. Brain Imaging Behav 4(2):121–131. https://doi.org/10.1007/s11682-010-9091-2
Takahashi Y, Kawakami M, Yamaguchi T, Idogawa Y, Tanabe S, Kondo K, Liu M (2019) Effects of leg motor imagery combined with electrical stimulation on plasticity of corticospinal excitability and spinal reciprocal inhibition. Front Neurosci 13:149. https://doi.org/10.3389/fnins.2019.00149 eCollection 2019
Hultborn H, Meunier S, Pierrot-Deseilligny E, Shindo M (1987) Changes in presynaptic inhibition of Ia fibres at the onset of voluntary contraction in man. J Physiol 389(1):757–772
Iles JF (1996) Evidence for cutaneous and corticospinal modulation of presynaptic inhibition of Ia afferents from the human lower limb. J Physiol 491(1):197–207
Meunier S, Pierrot-Deseilligny E (1998) Cortical control of presynaptic inhibition of Ia afferents in humans. Exp Brain Res 119(4):415–426
Katz R, Meunier S, Pierrot-Deseilligny E (1988) Changes in presynaptic inhibition of Ia fibres in man while standing. Brain. 111(2):417–437
Sasaki Y, Bunno Y, Suzuki T, Urabe Y (2013) Influence of motor imagery of isometric activity of the flexor hallucis brevis on the excitability of the spinal neural function. Rigakuryoho Kagaku 28(5):673–676
Maeda A, Fukumoto Y, Bunno Y, Suzuki T (2017) Influence on the excitability of spinal neural function of motor imagery in the thumb and index finger opposition position with orthosis. J Kansai Phys Ther 17:97–103
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Ethical information
All tests were performed at the Tokyo Bay Rehabilitation Hospital. All participants provided written, informed consent prior to enrolment. The procedures complied with the Declaration of Helsinki.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nakayama, H., Kawakami, M., Takahashi, Y. et al. The changes in spinal reciprocal inhibition during motor imagery in lower extremity. Neurol Sci 42, 3813–3820 (2021). https://doi.org/10.1007/s10072-021-05054-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-021-05054-z