Abstract
Objective
The hexanucleotide repeat expansion in C9orf72 is an associated genetic cause in amyotrophic lateral sclerosis (ALS) and frontotemporal dementia (FTD). In the “ALS/FTD” spectrum prevails clinical heterogeneity and an in vivo knowledge of the underling brain dysfunction in patients carrying C9orf72 mutation remain limited and only described at group level. The study aimed to assess the brain metabolic alterations characterizing patients with C9orf72 mutation using FDG-PET in single individuals.
Methods
We applied a validated statistical parametric mapping (SPM) voxel-based procedure for FDG-PET data to obtain maps of brain relative hypometabolism and hypermetabolism at single-subject level in six FTD/ALS patients carrying the C9orf72 mutation.
Results
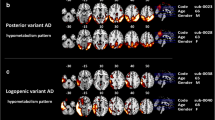
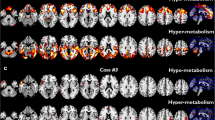
Clinical diagnoses classified the patients as right semantic variant of frontotemporal dementia (one case, C9svFTD), behavioral variant of frontotemporal dementia (two cases, C9bvFTD), and bulbar amyotrophic lateral sclerosis (three cases, C9bALS). The FDG-PET SPM revealed a prevalent frontal hypometabolism in C9bvFTD cases, and right temporal polar and lateral involvement in C9svFTD, consistent with the clinical diagnosis. There was a quite comparable occipital and cerebellar hypermetabolism in these cases. The three C9bALS patients showed variable patterns of hypo- and hypermetabolism.
Conclusions
The present work is the first in vivo FDG-PET study showing the heterogeneous patterns of brain regional hypo- and hypermetabolism in single patients sharing C9orf72 mutation. Brain hypometabolism was consistent with the clinical phenotypes, supporting the diagnostic importance of neuroimaging functional biomarkers to capture at single-subject level specific brain dysfunction.

Similar content being viewed by others
References
Renton AE, Majounie E, Waite A, Simón-Sánchez J, Rollinson S, Gibbs JR, Schymick JC, Laaksovirta H, van Swieten J, Myllykangas L, Kalimo H, Paetau A, Abramzon Y, Remes AM, Kaganovich A, Scholz SW, Duckworth J, Ding J, Harmer DW, Hernandez DG, Johnson JO, Mok K, Ryten M, Trabzuni D, Guerreiro RJ, Orrell RW, Neal J, Murray A, Pearson J, Jansen IE, Sondervan D, Seelaar H, Blake D, Young K, Halliwell N, Callister JB, Toulson G, Richardson A, Gerhard A, Snowden J, Mann D, Neary D, Nalls MA, Peuralinna T, Jansson L, Isoviita VM, Kaivorinne AL, Hölttä-Vuori M, Ikonen E, Sulkava R, Benatar M, Wuu J, Chiò A, Restagno G, Borghero G, Sabatelli M, ITALSGEN Consortium, Heckerman D, Rogaeva E, Zinman L, Rothstein JD, Sendtner M, Drepper C, Eichler EE, Alkan C, Abdullaev Z, Pack SD, Dutra A, Pak E, Hardy J, Singleton A, Williams NM, Heutink P, Pickering-Brown S, Morris HR, Tienari PJ, Traynor BJ (2011) A hexanucleotide repeat expansion in C9ORF72 is the cause of chromosome 9p21-linked ALS-FTD. Neuron 72:257–268. https://doi.org/10.1016/j.neuron.2011.09.010
DeJesus-Hernandez M, Mackenzie IR, Boeve BF, Boxer AL, Baker M, Rutherford NJ, Nicholson AM, Finch NCA, Flynn H, Adamson J, Kouri N, Wojtas A, Sengdy P, Hsiung GYR, Karydas A, Seeley WW, Josephs KA, Coppola G, Geschwind DH, Wszolek ZK, Feldman H, Knopman DS, Petersen RC, Miller BL, Dickson DW, Boylan KB, Graff-Radford NR, Rademakers R (2011) Expanded GGGGCC hexanucleotide repeat in noncoding region of C9ORF72 causes chromosome 9p-linked FTD and ALS. Neuron 72:245–256. https://doi.org/10.1016/j.neuron.2011.09.011
Yokoyama JS, Sirkis DW, Miller BL (2014) C9ORF72 hexanucleotide repeats in behavioral and motor neuron disease: clinical heterogeneity and pathological diversity. Am J Neurodegener Dis 3:1–18
Cruts M, Gijselinck I, Van Langenhove T et al (2013) Current insights into the C9orf72 repeat expansion diseases of the FTLD/ALS spectrum. Trends Neurosci 36:450–459. https://doi.org/10.1016/j.tins.2013.04.010
Haeusler AR, Donnelly CJ, Periz G et al (2014) C9orf72 nucleotide repeat structures initiate molecular cascades of disease. Nature 507:1–20. https://doi.org/10.1038/nature13124.C9orf72
Couratier P, Corcia P, Lautrette G, Nicol M, Marin B (2017) ALS and frontotemporal dementia belong to a common disease spectrum. Rev Neurol (Paris) 173:273–279
Silani V, Ludolph A, Fornai F (2017) The emerging picture of ALS: a multisystem, not only a “motor neuron disease.”. In: Arch Ital Biol
Boeve BF, Boylan KB, Graff-Radford NR, DeJesus-Hernandez M, Knopman DS, Pedraza O, Vemuri P, Jones D, Lowe V, Murray ME, Dickson DW, Josephs KA, Rush BK, Machulda MM, Fields JA, Ferman TJ, Baker M, Rutherford NJ, Adamson J, Wszolek ZK, Adeli A, Savica R, Boot B, Kuntz KM, Gavrilova R, Reeves A, Whitwell J, Kantarci K, Jack CR Jr, Parisi JE, Lucas JA, Petersen RC, Rademakers R (2012) Characterization of frontotemporal dementia and/or amyotrophic lateral sclerosis associated with the GGGGCC repeat expansion in C9ORF72. Brain 135:765–783. https://doi.org/10.1093/brain/aws004
Cistaro A, Pagani M, Montuschi A, Calvo A, Moglia C, Canosa A, Restagno G, Brunetti M, Traynor BJ, Nobili F, Carrara G, Fania P, Lopiano L, Valentini MC, Chiò A (2014) The metabolic signature of C9ORF72-related ALS: FDG PET comparison with nonmutated patients. Eur J Nucl Med Mol Imaging 41:844–852. https://doi.org/10.1007/s00259-013-2667-5
Van Laere K, Vanhee A, Verschueren J et al (2014) Value of 18Fluorodeoxyglucose–positron-emission tomography in amyotrophic lateral sclerosis: a prospective study. JAMA Neurol 71:553–561
Devenney E, Hornberger M, Irish M, Mioshi E, Burrell J, Tan R, Kiernan MC, Hodges JR (2014) Frontotemporal dementia associated with the C9ORF72 mutation: a unique clinical profile. JAMA Neurol 71:331–339. https://doi.org/10.1001/jamaneurol.2013.6002
Perani D, Della Rosa PA, Cerami C, Gallivanone F, Fallanca F, Vanoli EG, Panzacchi A, Nobili F, Pappatà S, Marcone A, Garibotto V, Castiglioni I, Magnani G, Cappa SF, Gianolli L, EADC-PET Consortium (2014) Validation of an optimized SPM procedure for FDG-PET in dementia diagnosis in a clinical setting. NeuroImage Clin 6:445–454. https://doi.org/10.1016/j.nicl.2014.10.009
Perani D, Cerami C, Caminiti SP, Santangelo R, Coppi E, Ferrari L, Pinto P, Passerini G, Falini A, Iannaccone S, Cappa SF, Comi G, Gianolli L, Magnani G (2016) Cross-validation of biomarkers for the early differential diagnosis and prognosis of dementia in a clinical setting. Eur J Nucl Med Mol Imaging 43:499–508. https://doi.org/10.1007/s00259-015-3170-y
Cerami C, Della Rosa PA, Magnani G, Santangelo R, Marcone A, Cappa SF, Perani D (2015) Brain metabolic maps in mild cognitive impairment predict heterogeneity of progression to dementia. NeuroImage Clin 7:187–194. https://doi.org/10.1016/j.nicl.2014.12.004
Cerami C, Dodich A, Lettieri G, Iannaccone S, Magnani G, Marcone A, Gianolli L, Cappa SF, Perani D (2016) Different FDG-PET metabolic patterns at single-subject level in the behavioral variant of fronto-temporal dementia. Cortex 83:101–112. https://doi.org/10.1016/j.cortex.2016.07.008
Cerami C, Dodich A, Greco L, Iannaccone S, Magnani G, Marcone A, Pelagallo E, Santangelo R, Cappa SF, Perani D (2017) The role of single-subject brain metabolic patterns in the early differential diagnosis of primary progressive aphasias and in prediction of progression to dementia. J Alzheimers Dis 55:183–197
Caminiti SP, Alongi P, Majno L, Volontè MA, Cerami C, Gianolli L, Comi G, Perani D (2017) Evaluation of an optimized [18F]fluoro-deoxy-glucose positron emission tomography voxel-wise method to early support differential diagnosis in atypical Parkinsonian disorders. Eur J Neurol 24:687–e26
Iaccarino L, Sala A, Caminiti SP, Perani D (2017) The emerging role of PET imaging in dementia. F1000Research 6:1830
Ratti A, Corrado L, Castellotti B et al (2012) C9ORF72 repeat expansion in a large Italian ALS cohort: evidence of a founder effect. Neurobiol Aging 33:2528–25e7
Varrone A, Asenbaum S, Vander Borght T, Booij J, Nobili F, Någren K, Darcourt J, Kapucu OL, Tatsch K, Bartenstein P, van Laere K, European Association of Nuclear Medicine Neuroimaging Committee (2009) EANM procedure guidelines for PET brain imaging using [18F] FDG, version 2. Eur J Nucl Med Mol Imaging 36:2103–2110. https://doi.org/10.1007/s00259-009-1264-0
Della Rosa PA, Cerami C, Gallivanone F et al (2014) A standardized [18F]-FDG-PET template for spatial normalization in statistical parametric mapping of dementia. Neuroinformatics 12:575–593
Friston KJ, Holmes AP, Worsley KJ, Poline JP, Frith CD, Frackowiak RSJ (1994) Statistical parametric maps in functional imaging: a general linear approach. Hum Brain Mapp 2:189–210
Cistaro A, Valentini MC, Chiò A, Nobili F, Calvo A, Moglia C, Montuschi A, Morbelli S, Salmaso D, Fania P, Carrara G, Pagani M (2012) Brain hypermetabolism in amyotrophic lateral sclerosis: a FDG PET study in ALS of spinal and bulbar onset. Eur J Nucl Med Mol Imaging 39:251–259
Khan BK, Yokoyama JS, Takada LT, et al (2012) Atypical, slowly progressive behavioural variant frontotemporal dementia associated with C9ORF72 hexanucleotide expansion. https://doi.org/10.1136/jnnp-2011-301883
Ishihara K, Araki S, Ihori N, Shiota JI, Kawamura M, Nakano I (2006) An autopsy case of frontotemporal dementia with severe dysarthria and motor neuron disease showing numerous basophilic inclusions. Neuropathology 26:447–454. https://doi.org/10.1111/j.1440-1789.2006.00717.x
Strong MJ, Abrahams S, Goldstein LH et al (2017) Amyotrophic lateral sclerosis - frontotemporal spectrum disorder (ALS-FTSD): Revised diagnostic criteria. Amyotroph Lateral Scler Front Degener. https://doi.org/10.1080/21678421.2016.1267768
Portet F, Cadilhac C, Touchon J, Camu W (2001) Cognitive impairment in motor neuron disease with bulbar onset. Amyotroph Lateral Scler Other Mot Neuron Disord 2:23–29. https://doi.org/10.1080/146608201300079382
Origone P, Accardo J, Verdiani S, Lamp M, Arnaldi D, Bellone E, Picco A, Morbelli S, Mandich P, Nobili F (2015) Neuroimaging features in C9orf72 and TARDBP double mutation with FTD phenotype. Neurocase 21:529–534
Levy JP, Bocti C, Elie D, et al (2018) Bifrontal hypermetabolism on brain FDG-PET in a case of C9orf72-related behavioral variant of frontotemporal dementia. J Neuropsychiatry Clin Neurosci appi-neuropsych
Josephs KA, Whitwell JL, Knopman DS, Boeve BF, Vemuri P, Senjem ML, Parisi JE, Ivnik RJ, Dickson DW, Petersen RC, Jack CR (2009) Two distinct subtypes of right temporal variant frontotemporal dementia. Neurology 73:1443–1450. https://doi.org/10.1212/WNL.0b013e3181bf9945
Schroeter ML, Laird AR, Chwiesko C, Deuschl C, Schneider E, Bzdok D, Eickhoff SB, Neumann J (2014) Conceptualizing neuropsychiatric diseases with multimodal data-driven meta-analyses - the case of behavioral variant frontotemporal dementia. Cortex 57:22–37. https://doi.org/10.1016/j.cortex.2014.02.022
Tan RH, Devenney E, Dobson-Stone C, Kwok JB, Hodges JR, Kiernan MC, Halliday GM, Hornberger M (2014) Cerebellar integrity in the amyotrophic lateral sclerosis - frontotemporal dementia continuum. PLoS One 9:e105632. https://doi.org/10.1371/journal.pone.0105632
Mackenzie IRA, Frick P, Neumann M (2014) The neuropathology associated with repeat expansions in the C9ORF72 gene. Acta Neuropathol 127:347–357. https://doi.org/10.1007/s00401-013-1232-4
Cooper-Knock J, Kirby J, Highley R, Shaw PJ (2015) The spectrum of C9orf72-mediated neurodegeneration and amyotrophic lateral sclerosis. Neurotherapeutics 12:326–339. https://doi.org/10.1007/s13311-015-0342-1
Rohrer JD, Isaacs AM, Mizlienska S et al (2015) C9orf72 expansions in frontotemporal dementia and amyotrophic lateral sclerosis. Lancet Neurol 14:291–301. https://doi.org/10.1016/S1474-4422(14)70233-9
Burrell JR, Halliday GM, Kril JJ, Ittner LM, Götz J, Kiernan MC, Hodges JR (2016) The frontotemporal dementia-motor neuron disease continuum. Lancet 388:919–931. https://doi.org/10.1016/S0140-6736(16)00737-6
Yokokura M, Mori N, Yagi S, Yoshikawa E, Kikuchi M, Yoshihara Y, Wakuda T, Sugihara G, Takebayashi K, Suda S, Iwata Y, Ueki T, Tsuchiya KJ, Suzuki K, Nakamura K, Ouchi Y (2011) In vivo changes in microglial activation and amyloid deposits in brain regions with hypometabolism in Alzheimer’s disease. Eur J Nucl Med Mol Imaging 38:343–351
Yamanaka K, Chun SJ, Boillee S, Fujimori-Tonou N, Yamashita H, Gutmann DH, Takahashi R, Misawa H, Cleveland DW (2008) Astrocytes as determinants of disease progression in inherited amyotrophic lateral sclerosis. Nat Neurosci 11:251–253
Turner MR, Cagnin A, Turkheimer FE, Miller CCJ, Shaw CE, Brooks DJ, Leigh PN, Banati RB (2004) Evidence of widespread cerebral microglial activation in amyotrophic lateral sclerosis: an [11C](R)-PK11195 positron emission tomography study. Neurobiol Dis 15:601–609
Grabert K, Michoel T, Karavolos MH et al (2016) Microglial brain region− dependent diversity and selective regional sensitivities to aging. Nat Neurosci 19:504
Byrne S, Elamin M, Bede P, Shatunov A, Walsh C, Corr B, Heverin M, Jordan N, Kenna K, Lynch C, McLaughlin RL, Iyer PM, O'Brien C, Phukan J, Wynne B, Bokde AL, Bradley DG, Pender N, al-Chalabi A, Hardiman O (2012) Cognitive and clinical characteristics of patients with amyotrophic lateral sclerosis carrying a C9orf72 repeat expansion: a population-based cohort study. Lancet Neurol 11:232–240. https://doi.org/10.1016/S1474-4422(12)70014-5
Cooper-Knock J, Shaw PJ, Kirby J (2014) The widening spectrum of C9ORF72-related disease; genotype/phenotype correlations and potential modifiers of clinical phenotype. Acta Neuropathol 127:333–345. https://doi.org/10.1007/s00401-014-1251-9
Adeli A, Savica R, Lowe VJ, Vemuri P, Knopman DS, DeJesus-Hernandez M, Rademakers R, Fields JA, Crum BA, Jack CR, Petersen RC, Boeve BF (2014) The GGGGCC repeat expansion in C9ORF72 in a case with discordant clinical and FDG-PET findings: PET trumps syndrome. Neurocase 20:110–120
Saint-Aubert L, Sagot C, Wallon D, Hannequin D, Payoux P, Nemmi F, Bezy C, Chauveau N, Campion D, Puel M, Chollet F, Pariente J (2014) A case of logopenic primary progressive aphasia with C9ORF72 expansion and cortical florbetapir binding. J Alzheimers Dis 42:413–420
Majounie E, Renton AE, Mok K, Dopper EG, Waite A, Rollinson S, Chiò A, Restagno G, Nicolaou N, Simon-Sanchez J, van Swieten J, Abramzon Y, Johnson JO, Sendtner M, Pamphlett R, Orrell RW, Mead S, Sidle KC, Houlden H, Rohrer JD, Morrison KE, Pall H, Talbot K, Ansorge O, Chromosome 9-ALS/FTD Consortium, French research network on FTLD/FTLD/ALS, ITALSGEN Consortium, Hernandez DG, Arepalli S, Sabatelli M, Mora G, Corbo M, Giannini F, Calvo A, Englund E, Borghero G, Floris GL, Remes AM, Laaksovirta H, McCluskey L, Trojanowski JQ, van Deerlin V, Schellenberg GD, Nalls MA, Drory VE, Lu CS, Yeh TH, Ishiura H, Takahashi Y, Tsuji S, le Ber I, Brice A, Drepper C, Williams N, Kirby J, Shaw P, Hardy J, Tienari PJ, Heutink P, Morris HR, Pickering-Brown S, Traynor BJ (2012) Frequency of the C9orf72 hexanucleotide repeat expansion in patients with amyotrophic lateral sclerosis and frontotemporal dementia: a cross-sectional study. Lancet Neurol 11:323–330
Martikainen MH, Gardberg M, Jansson L, Röyttä M, Rinne JO, Kaasinen V (2014) Brain 18F-FDG and 11C-PiB PET findings in two siblings with FTD/ALS associated with the C9ORF72 repeat expansion. Neurocase 20:150–157
Funding
This work was supported by the Italian Ministry of Health (Ricerca Finalizzata Progetto Reti Nazionale AD NET-2011-02346784) and a grant from IVASCOMAR Project (grant agreement no. CTN01_00177_165430).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 37 kb)
Rights and permissions
About this article
Cite this article
Castelnovo, V., Caminiti, S.P., Riva, N. et al. Heterogeneous brain FDG-PET metabolic patterns in patients with C9orf72 mutation. Neurol Sci 40, 515–521 (2019). https://doi.org/10.1007/s10072-018-3685-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-018-3685-7