Abstract
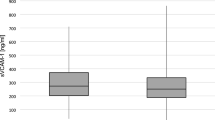
Angiogenesis has been implicated in the pathobiology of multiple sclerosis (MS). Osteopontin exerts a pro-angiogenetic effect and is increased in body fluid of MS patients. To evaluate the effect of 1 year natalizumab treatment on serum pro-angiogenic activity and on plasma osteopontin levels in relapsing (RR) MS patients. Ten RRMS patients scheduled for natalizumab treatment were enrolled and evaluated at baseline and after 1-year natalizumab treatment. Pro-angiogenic activity was assessed by a chick embryo chorioallantoic membrane assay (CAM), osteopontin levels were evaluated by an enzyme-linked immunosorbent assay. Plasma and serum samples of 10 treatment-naïve RRMS and 10 healthy controls (HCs) were used as controls of baseline evaluations. Both treatment-naïve and natalizumab scheduled RRMS patients had higher baseline vessel density (22.0 ± 3.9 and 22.5 ± 2.6, p < 0.0001) and higher osteopontin levels (65.7 ± 24.3 ng/ml and 65.9 ± 16.6 ng/ml, p = 0.019 and p = 0.029, respectively) than HCs (9.0 ± 2.2; 48.5 ± 7.8 ng/ml, respectively). Baseline osteopontin levels and vessel density were significantly correlated (rs = 0.373, p = 0.043). After 1 year of treatment, the number of vessels and the osteopontin levels, were significantly reduced (11.9 ± 2.1, p < 0.005; 49.3 ± 20.0 ng/ml, p = 0.028). Our results suggest that natalizumab could exert its anti-inflammatory properties also by inhibiting the angiogenetic mechanisms in RRMS patients.





Similar content being viewed by others
References
Girolamo F, Coppola C, Ribatti D, Trojano M (2014) Angiogenesis in multiple sclerosis and experimental autoimmune encephalomyelitis. Acta Neuropathol Commun 2(1):84. https://doi.org/10.1186/s40478-014-0084-z
Claudio L, Raine CS, Brosnan CF (1995) Evidence of persistent blood–brain barrier abnormalities in chronic-progressive multiple sclerosis. Acta Neuropathol 90(3):228–238. https://doi.org/10.1007/BF00296505
Kirk J, Plumb J, Mirakhur M, McQuaid S (2003) Tight junctional abnormality in multiple sclerosis white matter affects all calibers of vessel and is associated with blood–brain barrier leakage and active demyelination. J Pathol 201(2):319–327. https://doi.org/10.1002/path.1434
van Horssen J, Bö L, Vos CM, Virtanen I, de Vries HE (2005) Basement membrane proteins in multiple sclerosis-associated inflammatory cuffs: potential role in influx and transport of leukocytes. J Neuropathol Exp Neurol 64(8):722–729. https://doi.org/10.1097/01.jnen.0000173894.09553.13
Alvarez JI, Cayrol R, Prat A (2011) Disruption of central nervous system barriers in multiple sclerosis. Biochim Biophys Acta 812:252–264
Clauss M, Gerlach M, Gerlach H, Brett J, Wang F, Familletti PC, Pan YC, Olander JV, Connolly DT, Stern D (1990) Vascular permeability factor: a tumor-derived polypeptide that induces endothelial cell and monocyte procoagulant activity, and promotes monocyte migration. J Exp Med 172(6):1535–1545. https://doi.org/10.1084/jem.172.6.1535
Ribatti D, Iaffaldano P, Marinaccio C, Trojano M (2016) First evidence of in vivo pro-angiogenic activity of cerebrospinal fluid samples from multiple sclerosis patients. Clin Exp Med 16(1):103–107. https://doi.org/10.1007/s10238-014-0334-1
Junaid A, Moon MC, Harding GE, Zahradka P (2007) Osteopontin localizes to the nucleus of 293 cells and associates with p1-like kinase-1. Am J physiol Cell Physiol 292(2):c919–c926. https://doi.org/10.1152/ajpcell.00477.2006
Steinman L (2009) A molecular trio in relapse and remission in multiple sclerosis. Nat Rev Immunol 9(6):440–447. https://doi.org/10.1038/nri2548
Vogt M, Lopatinskaya L, Smits M, Polman CH, Nagelkerken L (2008) Elevated osteopontin levels in active relapsing-remitting multiple sclerosis. Ann Neurol 53:819–822
Comabella M, Pericot I, Goertsches R, Nos C, Castillo M, Blas Navarro J, Río J, Montalban X (2005) Plasma osteopontin levels in multiple sclerosis. J Neuroimmunol 158(1-2):231–239. https://doi.org/10.1016/j.jneuroim.2004.09.004
Iaffaldano P, Ruggieri M, Viterbo RG, Mastrapasqua M, Trojano M (2014) The improvement of cognitive functions is associated with a decrease of plasma osteopontin levels in natalizumab treated relapsing multiple sclerosis. Brain Behav Immun 35:176–181. https://doi.org/10.1016/j.bbi.2013.08.009
Chabas D, Baranzini SE, Mitchell D, Bernard CC, Rittling SR, Denhardt DT, Sobel RA, Lock C, Karpuj M, Pedotti R, Heller R, Oksenberg JR, Steinman L (2001) The influence of the proinflammatory cytokine, osteopontin, on autoimmune demyelinating disease. Science 294(5547):1731–1735. https://doi.org/10.1126/science.1062960
Polman CH, O'Connor PW, Havrdova E, Hutchinson M, Kappos L, Miller DH, Phillips JT, Lublin FD, Giovannoni G, Wajgt A, Toal M, Lynn F, Panzara MA, Sandrock AW, AFFIRM Investigators (2006) A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med 354(9):899–910. https://doi.org/10.1056/NEJMoa044397
Rudick RA, Stuart WH, Calabresi PA, Confavreux C, Galetta SL, Radue EW, Lublin FD, Weinstock-Guttman B, Wynn DR, Lynn F, Panzara MA, Sandrock AW, SENTINEL Investigators (2006) Natalizumab plus interferon beta-1a for relapsing multiple sclerosis. N Engl J Med 354(9):911–923. https://doi.org/10.1056/NEJMoa044396
Iaffaldano P, Viterbo RG, Paolicelli D, Lucchese G, Portaccio E, Goretti B, Direnzo V, D'Onghia M, Zoccolella S, Amato MP, Trojano M (2012) Impact of natalizumab on cognitive performances and fatigue in relapsing multiple sclerosis: a prospective, open-label, two years observational study. PLoS One 7(4):e35843. https://doi.org/10.1371/journal.pone.0035843
Khademi M, Bornsen L, Rafatnia F, Andersson M, Brundin L, Piehl F, Sellebjerg F, Olsson T (2009) The effects of natalizumab on inflammatory mediators in multiple sclerosis: prospects for treatment-sensitive biomarkers. Eur J Neurol 16(4):528–536. https://doi.org/10.1111/j.1468-1331.2009.02532.x
O'Connor PW, Goodman A, Kappos L, Lublin FD, Miller DH, Polman C, Rudick RA, Aschenbach W, Lucas N (2011) Disease activity return during natalizumab treatment interruption in patients with multiple sclerosis. Neurology 76(22):1858–1865. https://doi.org/10.1212/WNL.0b013e31821e7c8a
Cohen M, Maillart E, Tourbah A et al (2014) Switching from natalizumab to fingolimod in multiple sclerosis: a French prospective study. JAMA Neurol 71(4):436–441
Fox RJ, Cree BA, De Sèze J et al (2014) MS disease activity in RESTORE: a randomized 24-week natalizumab treatment interruption study. Neurology 82(17):1491–1498. https://doi.org/10.1212/WNL.0000000000000355
Iaffaldano P, Lucisano G, Pozzilli C, Brescia Morra V, Ghezzi A, Millefiorini E, Patti F, Lugaresi A, Zimatore GB, Marrosu MG, Amato MP, Bertolotto A, Bergamaschi R, Granella F, Coniglio G, Tedeschi G, Sola P, Lus G, Ferrò MT, Iuliano G, Corea F, Protti A, Cavalla P, Guareschi A, Rodegher M, Paolicelli D, Tortorella C, Lepore V, Prosperini L, Saccà F, Baroncini D, Comi G, and Maria Trojano on behalf of the Italian iMed-Web database (2015) Fingolimod versus interferon beta/glatiramer acetate after natalizumab suspension in multiple sclerosis. Brain 138(pt 11):3275–3286. https://doi.org/10.1093/brain/awv260
Iaffaldano P, Viterbo RG, Trojano M (2016) Natalizumab discontinuation is associated with a rebound of cognitive impairment in multiple sclerosis patients. J Neurol 263(8):1620–1625. https://doi.org/10.1007/s00415-016-8177-1
Podar K, Zimmerhackl A, Fulciniti M, Tonon G, Hainz U, Tai YT, Vallet S, Halama N, Jäger D, Olson DL, Sattler M, Chauhan D, Anderson KC (2011) The selective adhesion molecule inhibitor natalizumab decreases multiple myeloma cell growth in the bone marrow microenvironment: therapeutic implications. Br J Haematol 155(4):438–448. https://doi.org/10.1111/j.1365-2141.2011.08864.x
Ribatti D (2008) Chick embryo chorioallantoic membrane as a useful tool to study angiogenesis. Int Rev Cell Mol Biol 270:181–224. https://doi.org/10.1016/S1937-6448(08)01405-6
Ribatti D, Nico B, Vacca A, Presta M (2006) The gelatin sponge-chorioallantoic membrane assay. Nat Protoc 1(1):85–91. https://doi.org/10.1038/nprot.2006.13
Proescholdt MA, Jacobson S, Tresser N, Oldfield EH, Merrill MJ (2002) Vascular endothelial growth factor is expressed in multiple sclerosis plaques and can induce inflammatory lesions in experimental allergic encephalomyelitis rats. J Neuropathol Exp Neurol 61(10):914–925. https://doi.org/10.1093/jnen/61.10.914
Graumann U, Reynolds R, Steck AJ, Schaeren-Wiemers N (2003) Molecular changes in normal appearing white matter in multiple sclerosis are characteristic of neuroprotective mechanisms against hypoxic insult. Brain Pathol 13(4):554–573
Theoharides TC, Konstantinidou AD (2007) Corticotropin-releasing hormone and the blood-brain-barrier. Front Biosci 12(1):1615–1628. https://doi.org/10.2741/2174
Sinclair C, Mirakhur M, Kirk J, Farrell M, McQuaid S (2005) Up-regulation of osteopontin and alphaBeta-crystallin in the normal-appearing white matter of multiple sclerosis: an immunohistochemical study utilizing tissue microarrays. Neuropathol Appl Neurobiol 31(3):292–230. https://doi.org/10.1111/j.1365-2990.2004.00638.x
Bornsen L, Khademi M, Olsson T, Sørensen PS, Sellebjerg F (2011) Osteopontin concentrations are increased in cerebrospinal fluid during attacks of multiple sclerosis. Mult Scler 17(1):32–42. https://doi.org/10.1177/1352458510382247
Chakraborthy G, Jain S, Kundu GC (2008) Osteopontin promotes vascular endothelial growth factor–dependent breast tumor growth and angiogenesis via autocrine and paracrine mechanisms. Cancer Res 68(1):152–161. https://doi.org/10.1158/0008-5472.CAN-07-2126
Li XD, Chen J, Ruan CC, Zhu DL, Gao PJ (2012) Vascular endothelial growth factor-induced osteopontin extression emdiates vasculr inflammation and neointima formation via Flt-1 in adventitial fibroblasts. Arterioscler Thromb Vasc Biol 32(9):2250–2258. https://doi.org/10.1161/ATVBAHA.112.255216
Ribatti D, Crivellato E (2009) Immune cells and angiogenesis. J Cell Mol Med 13(9A):2822–2833. https://doi.org/10.1111/j.1582-4934.2009.00810.x
Leali D, Dell'Era P, Stabile H, Sennino B, Chambers AF, Naldini A, Sozzani S, Nico B, Ribatti D, Presta M (2003) Osteopontin (Eta-1) and fibroblast growth factor-2 cross-talk in angiogenesis. J Immunol 171(2):1085–1093. https://doi.org/10.4049/jimmunol.171.2.1085
Naldini A, Leali D, Pucci A, Morena E, Carraro F, Nico B, Ribatti D, Presta M (2006) Cutting edge: IL-1beta mediates the proangiogenic activity of osteopontin-activated human monocytes. J Immunol 177(7):4267–4270. https://doi.org/10.4049/jimmunol.177.7.4267
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest statement
PI has served on scientific advisory boards for Biogen Idec, and has received funding for travel and/or speaker honoraria from Sanofi-Aventis, Biogen Idec, Teva and Novartis. MT has received honoraria for consultancy or speaking from Biogen, Sanofi-Aventis, Merck Serono, and Bayer-Schering and research grants from Merck Serono, Biogen and Novartis. DR declares no conflict of interest.
Ethical approval
The study was approved by the Ethics Committee of the University of Bari Medical School.
Informed consent
Written informed consent was obtained by all patients before the start of natalizumab (NTZ).
Rights and permissions
About this article
Cite this article
Iaffaldano, P., Ribatti, D. & Trojano, M. Natalizumab reduces serum pro-angiogenic activity in MS patients. Neurol Sci 39, 725–731 (2018). https://doi.org/10.1007/s10072-018-3266-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-018-3266-9