Abstract
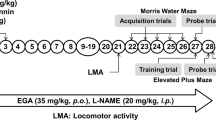
Oxidative stress leads to complex biochemical alterations, and has been implicated in the progressive loss of learning and memory. Supplementing and boosting the endogenous antioxidant defense system could impede the progression of various types of neurodegeneration. In the present study, we have investigated the neuroprotective efficacy of a low-dose combination of certain promising and powerful natural antioxidants in an experimental model of cognitive impairment. Combined pretreatment with the extract of Nardosatchys jatamansi (N), crocetin (C) and selenium (Se) as sodium selenite (N, 200 mg/kg + C, 25 μg/kg + Se, 0.05 mg/kg body weight) for 15 days led to improved behavioral outcomes in streptozotocin (STZ)-induced cognitive impairment in rats. While intracerebroventricular (ICV) infusion of STZ resulted in the significant elevation of markers of oxidative stress and depletion of endogenous antioxidant defense system in the vehicle-pretreated group, these markers of oxidative stress and antioxidant enzymatic as well as non-enzymatic defense lines were attenuated in the group pretreated with the combination of antioxidants (NCSe). NCSe pretreatment markedly improved the performance of animals in passive avoidance test and Morris water maze (MWM) tasks, significantly reduced the level of TBARS, and elevated the content of glutathione and activities of antioxidant enzymes (glutathione peroxidase, glutathione-S-transferase and catalase). Our study reflects the synergistic potential of the above combination and concludes that a multimodal approach could be beneficial rather than a singular intervention.






Similar content being viewed by others
References
Halliwell B (1992) Reactive oxygen species and the central nervous system. J Neurochem 59:1609–1623
Coyle JT, Puttfarcken P (1993) Oxidative stress, glutamate and neurodegenerative disorders. Science 262:689–695
Olanow CW (1993) A radical hypothesis for neurodegeneration. Trends Neurosci 16:439–444
Mate′s JM, Pe′rez-Go′mez C, Castro C (1999) Antioxidant enzymes and human diseases. Clin Biochem 32:595–603
Alexi T, Borlongan CV, Faull RLM, Williams CE, Clark RG, Gluckman PD, Hughes PE (2000) Neuroprotective strategies for basal ganglia degeneration: Parkinson’s and Huntington’s diseases. Prog Neurobiol 60:409–470
Halliwell B, Gutteridge JMC (1999) Free radicals in biology and medicine, 3rd edn. Oxford University Press, New York
Urano S, Asai Y, Makabe S, Matsuo M, Izumiyama N, Ohtsubo K, Endo T (1997) Oxidative injury of synapse and alteration of antioxidative defense systems in rats, and its prevention by vitamin E. Eur J Biochem 245:64–70
Yamamoto T, Yuki S, Watanabe T, Mitsuka M, Saito KI, Kogure K (1997) Delayed neuronal death prevented by inhibition of increased hydroxyl radical formation in a transient cerebral ischemia. Brain Res 762:240–242
Bastianetto S, Ramassamy C, Poirier J, Quiron R (1999) Dehydroepiandrosterone DHEA protects hippocampal cells from oxidative stress-induced damage. Brain Res Mol Brain Res 66:35–41
Blokland A, Jolles J (1993) Spatial learning deficit and reduced hippocampal ChAT activity in rats after an ICV injection of streptozotocin. Pharmacol Biochem Behav 44:491–494
Lannert H, Hoyer S (1998) Intracerebroventricular administration of streptozotocin causes long-term diminutions in learning and memory abilities and in cerebral energy metabolism in adult rats. Behav Neurosci 112:1199–1208
Chatterjee A, Prakashi SC (1997) The treatise on Indian medicinal plants, vol 5. National Institute of Science Communication, New Delhi, pp 99–100
Arora RB (1965) Nardostachys jatamansi—a chemical, pharmacological and clinical appraisal. Monograph Special Series, vol 51. ICMR, New Delhi
Rastogi BR, Mehrotra K (1990) Compendium of Indian medicinal plants, 4th edn. Public Information Directorate, CSIR, New Delhi, pp 286–287
Tripathi YB, Tripathi E, Upadhyaya A (1996) Antilipid peroxidative property of Nardostachys jatamansi. Indian J Exp Biol 34:1150–1161
Joshi H, Parle M (2006) Nardostachys jatamansi improves learning and memory in mice. J Med Food 9:113–118
Ahmad M, Yousuf S, Khan MB, Hoda MN, Ahmad AS, Ansari MA, Ishrat T, Agrawal AK, Islam F (2006) Attenuation by Nardostachys jatamansi of 6-hydroxydopamine-induced Parkinsonism in rats: behavioral, neurochemical, and immunohistochemical studies. Pharmacol Biochem Behav 83:150–160
Salim S, Ahmad M, Zafar KS, Ahmad AS, Islam F (2003) Protective effect of Nardostachys jatamansi in rat cerebral ischemia. Pharmacol Biochem Behav 74:481–486
Singh A, Kumar A, Duggal S (2009) Nardostachys jatamansi DC. Potential herb with CNS effects. J Pharm Res Health Care 1:276–290
Rios JL, Recio MC, Ginger RM, Manz S (1996) An update review of saffron and its active constituents. Phytother Res 10:189–193
Hosseinzadeh H, Khosravan V (2002) Anticonvulsant effects of aqueous and ethanolic extracts of Crocus sativus L. stigmas in mice. Arch Irn Med 5:44–47
Hosseinzadeh H, Younesi HM (2002) Antinociceptive and anti-inflammatory effects of Crocus sativus L. stigma and petal extracts in mice. BMC Pharmacol 2:1–8
Hosseinzadeh H, Karimi G, Niapoor M (2004) Antidepressant effects of Crocus sativus stigma extracts and its constituents, crocin and safranal, in mice. Acta Hort ISHS 650:435–445
Abdullaev FJ (1993) Biological effects of saffron. Biofactors 4:83–86
Escribano J, Alonso GL, Coca-Prados M, Fernandez JA (1996) Crocin, safranal and picrocrocin from saffron (Crocus sativus L.) inhibit the growth of human cancer cells in vitro. Cancer Lett 100:23–30
Zhang YX, Sugiura M, Saito H, Shoyama Y (1994) Acute effects of Crocus sativus L. on passive avoidance performance in mice. Biol Pharmacol Bull 17:217–221
Abe K, Sugiura M, Ymaguchi S, Shoyama Y, Saito H (1999) Saffron extract prevents acetaldehyde-induced inhibition of long-term potentiation in the rat dentate gyrus in vivo. Brain Res 851:287–289
Papandreou MA, Kanakis CD, Polissiou MG, Efthimiopoulos S, Cordopatis P, Margarity M, Lamari FN (2006) Inhibitory activity on amyloid-beta aggregation and antioxidant properties of Crocus sativus stigmas extract and its crocin constituents. J Agric Food Chem 54:8762–8768
Ahmad AS, Ansari MA, Ahmad M, Saleem S, Yousuf S, Hoda MN, Islam F (2005) Neuroprotection by crocetin in a hemi-parkinsonian rat model. Pharmacol Biochem Behav 81:805–813
Schwarz K, Folz CM (1957) Selenium as an integral part of factor 3 against dietary necrotic liver degeneration. Nutrition 15:255–264
Brauer AU, Savaskan NE (2004) Molecular actions of selenium in the brain: neuroprotective mechanisms of an essential trace element. Rev Neurosci 15:1–19
Harman D (1993) Free radicals and age-related diseases. In: Pal YB (ed) Free radicals in aging. CRC Press, Boca Raton, pp 205–222
Lee SR, Bar-Noy S, Kwon J, Levine RL, Stadtman TC, Rhee SG (2000) Mammalian thioredoxin reductase: oxidation of the C-terminal cysteine/selenocysteine active site forms a thioselenide, and replacement of selenium with sulfur markedly reduces catalytic activity. Proc Natl Acad Sci USA 97:2521–2526
Ishrat T, Parveen K, Khan MM, Khuwaja G, Khan MB, Yousuf S, Ahmad A, Shrivastav P, Islam F (2009) Selenium prevents cognitive decline and oxidative damage in rat model of streptozotocin-induced experimental dementia of Alzheimer’s type. Brain Res 1281:117–127
Zafar KS, Siddiqui A, Sayeed I, Ahmad M, Salim S, Islam F (2003) Dose-dependent protective effect of selenium in rat model of Parkinson’s disease: neurobehavioral and neurochemical evidences. J Neurochem 84:438–446
Yousuf S, Atif F, Hoda N, Ahmad M, Saleem S, Ishrat T, Khan MB, Ahmad AS, Islam F (2007) Oral supplementation of Majun Baladar ameliorates antioxidant enzyme activities in cerebral ischaemic damage. Basic Clin Pharmacol Toxicol 101:246–453
Halliwell B, Gutteridge JMC (1984) Lipid peroxidation, oxygen radicals, cell damage and antioxidant therapy. Lancet 1:1396–1397
Westermarck T, Santavouri P (1984) Principles of antioxidant therapy in neuronal ceroid lipofuscinosis. Med Biol 62:148–151
Khan MB, Hoda MN, Yousuf S, Ishrat T, Ahmad M, Ahmad AS, Alavi SH, Haque N, Islam F (2006) Prevention of cognitive impairments and neurodegeneration by Khamira Abresham Hakim Arshad Wala. J Ethnopharmacol 108:68–73
Prabhu VM, Karandh SK, Rao A, Vidya PM, Sudhakar K (1994) Effect of Nardostachys jatamansi on biogenic amines and inhibitory amino acids on rat brain. Planta Med 60:114–217
Ishrat T, Khan MB, Hoda MN, Yousuf S, Ahmad M, Ansari MA, Ahmad AS, Islam F (2006) Coenzyme Q10 modulates cognitive impairment against intracerebroventricular injection of streptozotocin in rats. Behav Brain Res 171:9–16
Olariu A, Tran MH, Yamada K, Mizuno M, Hefco V, Nabeshima T (2001) Memory deficits and increased emotionality induced by β-amyloid 25–35 are correlated with the reduced acetylcholine release and altered phorbol dibutyrate binding in the hippocampus. J Neural Transm 108:1065–1079
Sharma M, Gupta YK (2003) Effect of alpha lipoic acid on intracerebroventricular streptozotocin model of cognitive impairment in rats. Eur Neuropsychopharm 13:241–247
Morris R (1984) Development of the water maze procedure for studying spatial learning in the rat. J Neurosci Methods 11:47–60
Gallagher M, Burwell R, Burchinal M (1993) Severity of spatial learning impairment in aging: development of a learning index for performance in the Morris water maze. Behav Neurosci 107:618–626
Utely HC, Bernheim F, Hochslein P (1967) Effect of sulfhydryl reagent on peroxidation in microsome. Arch Biochem Biophys 118:29–32
Islam F, Zia S, Sayeed I, Zafar KS, Ahmad AS (2002) Selenium induced alteration on lipids, lipid peroxidation, and thiol group in circadian rhythm centers of rat. Biol Trace Elem Res 90:1–12
Jollow DJ, Mitchell JR, Zampagloine N, Gillete JR (1974) Bromobenzene-induced liver necrosis: protective role of glutathione and evidence for 3, 4- bromobenzene oxide as the hepatotoxic metabolite. Pharmacology 11:151–169
Mohandas J, Marshall JJ, Duggi GG, Horvath JS, Tiller D (1984) Differential distribution of glutathione and glutathione related enzymes in rabbit kidneys: possible implication in analgesic neuropathy. Cancer Res 44:5086–5091
Habig WH, Pabst MJ, Jokoby B (1974) Glutathion-S-transferase: the first enzymatic step in mercapturic acid formation. J Biol Chem 249:7130–7139
Athar M, Mukhtar H, Bickers DR, Khan IU, Kalyanaraman B (1989) Evidence for the metabolism of tumor promoter organic hydroperoxides into free radicals by human carcinoma skin keratinocytes: an ESR-spin trapping study. Carcinogenesis 10:1499–1503
Stevens MJ, Obrosova I, Cao X, Van Huysen C, Greene DA (2000) Effects of DL-alpha-lipoic acid on peripheral nerve conduction, blood flow, energy metabolism, and oxidative stress in experimental diabetic neuropathy. Diabetes 49:1006–1015
Claiborne A (1985) Catalase activity. In: Greenwald RA (ed) CRC handbook of methods for oxygen radical research. CRC Press, Boca Raton, pp 283–284
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with Folin phenol reagent. J Biol Chem 193:265–275
Zs-Nagy I (1978) A membrane hypothesis of aging. J Theor Biol 75:189–195
Zs-Nagy I (1990) Dietary antioxidants and brain aging: hopes and facts. In: Ingram DK, Baker GT, Shock NW (eds) The potential for nutritional modulation of aging processes. Food and Nutrition Press, Trumbell, pp 379–399
Blokland A (1995) Acetylcholine: a neurotransmitter for learning and memory. Brain Res Brain Res Rev 21:285–300
Nitsch R, Hoyer S (1991) Local action of the diabetogenic drug, streptozotocin, on glucose and energy metabolism in rat brain cortex. Neurosci Lett 128:199–202
Hoyer S, Hennenberg N, Knapp S, Lannert H, Martin E (1996) Brain glucose metabolism is controlled by amplification and desensitization of the neuronal insulin receptor. Ann NY Acad Sci 777:374–379
Hoyer S (2000) Brain glucose and energy metabolism abnormalities in sporadic Alzheimer disease causes and consequences: an update. Exp Gerontol 35:1363–1372
Watt F (1996) Nuclear microscope analysis in Alzheimer’s and Parkinson’s disease: a review. Cell Mol Biol 42:17–26
Mattson MP, Chan SL (2001) Dysregulation of cellular calcium homeostasis in Alzheimer’s disease: bad genes and bad habits. J Mol Neurosci 17:205–224
Arlt S, Beisiegel U, Kontush A (2002) Lipid peroxidation in neurodegeneration: new insights into Alzheimer’s disease. Curr Opin Lipidol 13:289–294
Ansari MA, Ahmad AS, Ahmad M, Salim S, Yousuf S, Ishrat T, Islam F (2004) Selenium protects cerebral ischemia in rat brain mitochondria. Biol Trace Elem Res 101:73–86
Ahmad AS, Zia S, Sayeed I, Ansari MA, Ahmad M, Salim S, Yousuf S, Islam F (2005) Sodium selenite stimulates neurobehavior and neurochemical activities in rats. Biol Trace Elem Res 103:59–68
Li P, Yamakuni T, Matsunaga K, Kondo S, Ohizumi Y (2003) Nardosinone enhances nerve growth factor-induced neurite outgrowth in a mitogen-activated protein kinase- and protein kinase C-dependent manner in PC12D cells. J Pharmacol Sci 93:122–125
Acknowledgments
We are thankful to the Department of Ayurveda, Yoga and Naturopathy, Unani Siddha and Homeopathy (AYUSH) and the Ministry of Health and Family Welfare, Government of India, for financial assistance. The authors are thankful to Mr. Dharamvir and late Mr. Anil Kumar for help and cooperation.
Conflict of interest
We have no conflict of interest and certify hereby that this work has never been published.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Khan, M.B., Hoda, M.N., Ishrat, T. et al. Neuroprotective efficacy of Nardostachys jatamansi and crocetin in conjunction with selenium in cognitive impairment. Neurol Sci 33, 1011–1020 (2012). https://doi.org/10.1007/s10072-011-0880-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-011-0880-1