Abstract
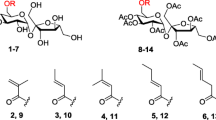
It has been proposed that the hydrophilic and/or lipophilic characteristics of fatty acid derivatives affect their antibacterial activities according to their ability to incorporate into the bacterial cell membrane. To verify this hypothesis, six kinds of lauric acid derivatives esterified with different non-fatty acid moieties were selected to confirm whether antibacterial activity from their precursor (i.e., lauric acid) is retained or lost. Three compounds, monolaurin, sucrose laurate, and erythorbyl laurate, exerted bacteriostatic and bactericidal effects against Gram-positive bacteria, while the others showed no inhibitory activity. Interestingly, the calculated log P (octanol–water partition coefficient) values of monolaurin, sucrose laurate, and erythorbyl laurate were − 4.122, − 0.686, and 3.670, respectively, relatively lower than those of the other compounds without antibacterial activity. Moreover, the hydrophilic-lipophilic balance values of the three compounds with antibacterial activity were higher than those of the other compounds, corresponding to the log P result.




Similar content being viewed by others
Change history
12 June 2018
In the original version of these 14 articles the reference list was unfortunately not represented according to the journal’s new bibliographical style, which should have been implemented from January 2018.
References
Altieri C, Bevilacqua A, Cardillo D, Sinigaglia M. Effectiveness of fatty acids and their monoglycerides against gram‐negative pathogens. Int J Food Sci Tech. 44: 359–366 (2009)
Arouri A, Mouritsen OG. Membrane-perturbing effect of fatty acids and lysolipids. Prog Lipid Res. 52: 130–140 (2013)
Bechert T, Steinrücke P, Guggenbichler J-P. A new method for screening anti-infective biomaterials. Nat Med. 6: 1053–1056 (2000)
Dayrit FM. The Properties of Lauric Acid and Their Significance in Coconut Oil. J Am Oil Chem Soc. 92: 1–15 (2015)
Desbois AP, Smith VJ. Antibacterial free fatty acids: activities, mechanisms of action and biotechnological potential. Appl Microbiol Biotechnol. 85: 1629–1642 (2010)
Friberg S, Larsson K, Sjoblom J. Food emulsions. 4th edn. CRC Press, Boca Raton, FL (2003)
Guo X, Rong Z, Ying X. Calculation of hydrophile–lipophile balance for polyethoxylated surfactants by group contribution method. J Colloid Interface Sci. 298: 441–450 (2006)
Hopkins AL, Keserü GM, Leeson PD, Rees DC, Reynolds CH. The role of ligand efficiency metrics in drug discovery. Nat Rev Drug Discov. 13: 105 (2014)
Hyldgaard M, Sutherland DS, Sundh M, Mygind T, Meyer RL. Antimicrobial Mechanism of Monocaprylate. Appl Environ Microbiol. 78: 2957–2965 (2012)
Kabara J, Vrable R, Jie MLK. Antimicrobial lipids: natural and synthetic fatty acids and monoglycerides. Lipids. 12: 753–759 (1977)
Kabara JJ, Swieczkowski DM, Conley AJ, Truant JP. Fatty acids and derivatives as antimicrobial agents. Antimicrob Agents Chemother. 2: 23–28 (1972)
Karmee SK. Lipase catalyzed synthesis of ester-based surfactants from biomass derivatives. Biofuels, Bioprod Biorefin. 2: 144–154 (2008)
Lieberman S, Enig MG, Preuss HG. A review of monolaurin and lauric acid: natural virucidal and bactericidal agents. Alternative and Complementary Therapies. 12: 310–314 (2006)
Luther M, Parry J, Moore J, Meng J, Zhang Y, Cheng Z, Yu LL. Inhibitory effect of Chardonnay and black raspberry seed extracts on lipid oxidation in fish oil and their radical scavenging and antimicrobial properties. Food Chem. 104: 1065–1073 (2007)
Magalhães L, Nitschke M. Antimicrobial activity of rhamnolipids against Listeria monocytogenes and their synergistic interaction with nisin. Food Control. 29: 138–142 (2013)
Meylan WM, Howard PH. Atom/fragment contribution method for estimating octanol–water partition coefficients. J Pharm Sci. 84: 83–92 (1995)
Nakatsuji T, Kao MC, Fang J-Y, Zouboulis CC, Zhang L, Gallo RL, Huang C-M. Antimicrobial property of lauric acid against Propionibacterium acnes: its therapeutic potential for inflammatory acne vulgaris. J Invest Dermatol. 129: 2480–2488 (2009)
Nobmann P, Bourke P, Dunne J, Henehan G. In vitro antimicrobial activity and mechanism of action of novel carbohydrate fatty acid derivatives against Staphylococcus aureus and MRSA. J Appl Microbiol. 108: 2152–2161 (2010)
Park K-M, Jo S-K, Yu H, Park J-Y, Choi SJ, Lee CJ, Chang P-S. Erythorbyl laurate as a potential food additive with multi-functionalities: Antibacterial activity and mode of action. Food Control. 86: 138–145 (2018)
Park K-M, Lee MJ, Jo S-K, Choi SJ, Lee J, Chang P-S. Erythorbyl laurate as a potential food additive with multi-functionalities: Interfacial characteristics and antioxidant activity. Food Chem. 215: 101–107 (2017)
Park K-M, Sung H, Lee J, Chang P-S. Lipase-catalysed synthesis of erythorbyl laurate in acetonitrile. Food Chem. 129: 59–63 (2011)
Pridmore A, Burch D, Lees P. Determination of minimum inhibitory and minimum bactericidal concentrations of tiamulin against field isolates of Actinobacillus pleuropneumoniae. Vet Microbiol. 151: 409–412 (2011)
Royer M, Nollet M, Catté M, Collinet M, Pierlot C. Towards a new universal way to describe the required hydrophilic lipophilic balance of oils using the phase inversion temperature of C10E4/n-octane/water emulsions. Colloids Surf Physicochem Eng Aspects. 536: 165–171 (2018)
Sabljic A, Guesten H, Hermens J, Opperhuizen A. Modeling octanol/water partition coefficients by molecular topology: chlorinated benzenes and biphenyls. Environ Sci Technol. 27: 1394–1402 (1993)
Watanabe Y, Ishido E, Fang X, Adachi S, Matsuno R. Oxidation kinetics of linoleic acid in the presence of saturated acyl l-ascorbate. J Am Oil Chem Soc. 82: 389–392 (2005)
Wiegand I, Hilpert K, Hancock RE. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat Protoc. 3: 163–175 (2008)
Yan Y, Bornscheuer UT, Schmid RD. Lipase-catalyzed synthesis of vitamin C fatty acid esters. Biotechnol Lett. 21: 1051–1054 (1999)
Yi B, Kim M-J, Lee J. Effects of emulsifier charges on the oxidative stability in oil-in-water emulsions under riboflavin photosensitization. Food Sci Biotechnol. 25: 1003–1009 (2016)
Zhao L, Zhang H, Hao T, Li S. In vitro antibacterial activities and mechanism of sugar fatty acid esters against five food-related bacteria. Food Chem. 187: 370–377 (2015)
Zheng CJ, Yoo JS, Lee TG, Cho HY, Kim YH, Kim WG. Fatty acid synthesis is a target for antibacterial activity of unsaturated fatty acids. FEBS Lett. 579: 5157–5162 (2005)
Acknowledgements
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT, & Future Planning (NRF-2017R1A2B4009230) and the Ministry of Education (NRF-R1A6A3A01012396).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Park, KM., Lee, S.J., Yu, H. et al. Hydrophilic and lipophilic characteristics of non-fatty acid moieties: significant factors affecting antibacterial activity of lauric acid esters. Food Sci Biotechnol 27, 401–409 (2018). https://doi.org/10.1007/s10068-018-0353-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10068-018-0353-x