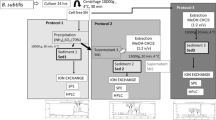
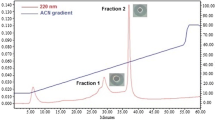
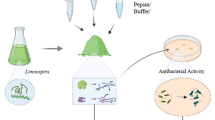
Abstract
Actinopyga lecanora, as a rich protein source was hydrolysed to generate antibacterial bioactive peptides using different proteolytic enzymes. Bromelain hydrolysate, after 1 h hydrolysis, exhibited the highestantibacterial activities against Pseudomonas aeruginosa, Pseudomonas sp., Escherichia coli and Staphylococcus aureus. Two dimensional fractionation strategies, using a semi-preparative RP-HPLC and an isoelectric-focusing electrophoresis, were applied for peptide profiling. Furthermore, UPLC-QTOF-MS was used for peptides identification; 12 peptide sequences were successfully identified. The antibacterial activity of purified peptides from A. lecanora on P. aeruginosa, Pseudomonas sp., E. coli and S. aureus was investigated. These identified peptides exhibited growth inhibition against P. aeruginosa, Pseudomonas sp., E. coli and S. aureus with values ranging from 18.80 to 75.30%. These results revealed that the A. lecanora would be used as an economical protein source for the production of high value antibacterial bioactive peptides.


Similar content being viewed by others
References
Sarmadi B, Ismail A, & Hamid, M. Antioxidant and angiotensin converting enzyme (ACE) inhibitory activities of cocoa (Theobroma cacao L.) autolysates. Food Res. Int. 44: 290–296(2011).
Kim S.-K, & Wijesekara, I. Development and biological activities of marine-derived bioactive peptides: A review. J Funct. Foods. 2: 1–9(2010).
Hancock R. E. W, & Sahl, H.-G. Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies. Nat Biotechnol. 24: 1551–1557(2006).
Gallo R. L, Murakami M, Ohtake, T, & Zaiou M. Biology and clinical relevance of naturally occurring antimicrobial peptides. J Allergy. Clin. Immunol. 110: 823–831(2002).
Zhang Y.-x., Zou, A.-h., Manchu, R.-g., Zhou, Y.-c., & Wang, S.-f. Purification and antimicrobial activity of antimicrobial protein from Brown-spotted Grouper, Epinephelus fario. Zool Res. 6627–632(2008).
Shahidi F, & Zhong Y. Bioactive peptides. J. AOAC. Int. 91: 914–931 (2008).
Ghanbari R, Ebrahimpour A, Abdul-Hamid A, Ismail A, & Saari N. Actinopyga lecanora hydrolysates as natural antibacterial agents. Int J. Mol. Sci. 13: 16796–16811(2012).
Church F C, Swaisgood H E, Porter D H, & Catignani G L. Spectrophotometric assay using o-Phthaldialdehyde for determination of proteolysis in milk and isolated milk proteins. J Dairy. Sci. 66: 1219–1227(1983).
Liu Z, Dong S, Xu J, Zeng M, Song H, & Zhao Y. Production of cysteine-rich antimicrobial peptide by digestion of oyster (Crassostrea gigas) with alcalase and bromelin. Food Control. 19: 231–235(2008).
Tang W, Zhang H, Wang L, & Qian H. Antimicrobial peptide isolated from ovalbumin hydrolysate by immobilized liposome-binding extraction. Eur Food. Res. Technol. 1–10(2013).
Nokihara K, Yamamoto R, Hazama M, Wakizawa O, & Nakamura S. Design and applications of a novel simultaneous multiple solid-phase peptide synthesizer, In R. Epton (Ed.) (pp. 445–448). Andover, UK: Intercept Limited(1992).
Nakajima Y, Ishibashi J, Yukuhiro F, Asaoka A, Taylor D, & Yamakawa, M. Antibacterial activity and mechanism of action of tick defensin against Gram-positive bacteria. Biochimicaet Biophysica Acta.1624: 125–130(2003).
Osman A, Goda H, Abdol-Hamid M, Badra S, & Otte, J. Antibacterial peptide generated by alkalase hydrolysis of goat whey. LWT- Food sci. technol. 65: 480–486 (2016).
Lam H.-T, Josserand J, Lion N, & Girault H H. Modeling the isoelectric focusing of peptides in an OFFGEL multicompartment cell. J. Proteome Res. 6: 1666–1676 (2007).
Hancock, R. E. W. Cationic peptides: effectors in innate immunity and novel antimicrobials. Lancet Infect. Dis. 1: 156–164(2001).
Dathe M, Nikolenko H, Meyer J, Beyermann M, & Bienert M. Optimization of the antimicrobial activity of magainin peptides by modification of charge. FEBS Letters. 501: 146–150(2001).
Sila A, Nedjar-Arroume N, Hedhili K, Chataigne G, Balti R, Nasri M, Dhulster P, & Bougatef A, Antibacterial peptides from barbel muscle protein hydrolysates: Activity against some pathogenic bacteria. LWT Food. Sci. Technol. 55: 183–188(2014).
Jang A, Jo C, Kang K.-S, & Lee M. Antimicrobial and human cancer cell cytotoxic effect of synthetic angiotensin-converting enzyme (ACE) inhibitory peptides. Food. Chem. 107: 327–336(2008).
Fjell C. D, Hiss J, AHancock, R. E. W, & Schneider G. Designing antimicrobial peptides: form follows function. Nat. Rev. Drug. Discov.11:3751(2012).
Brogden K. A. Antimicrobial peptides: pore formers or metabolic inhibitors in bacteria? Nat. Rev. Microbi. 3: 238–250(2005).
Hali, N.R.A, Yusof H. M, & Sarbon N.M. Functional and bioactive properties of fish protein hydrolysates and peptides: A comprehensive review. Trends Food. Sci. Technol. (2016).
Hwang C-F, Chen Y-A, Luo C, & Chiang W-D. Antioxidant and antibacterial activities of peptide fractions from flaxseed protein hydrolysed by protease from Bacillus altitudinis HK02. Int Food. Sci. Technol. 51: 681–869 (2016).
Conlon J. M, Al-Ghaferi N, Abraham B, & Leprince J. r. m. Strategies for transformation of naturally-occurring amphibian antimicrobial peptides into therapeutically valuable anti-infective agents. Methods. 42: 349–357(2007).
Aissaoui N, Chobert J-M, Haertlé T, Marzouki M-N, & Abidi F. Purification and biochemical characterization of a neutral serine protease from trichoderma harzianum. use in antibacterial peptide production from a fish by-product hydrolysate. Appl. Biochem. Biotechnol. 182: 831–845(2017).
Dennison S. R, Wallace J, Harris F, & Phoenix D. A. Amphiphilic α-Helical antimicrobial peptides and their structure/function relationships. Protein. Pept. Lett. 12: 31–39 (2005).
Park C. B, Yi K.-S, MatsuzakiK, Kim M. S, & Kim S. C. Structure-activity analysis of buforin II, a histone H2A-derived antimicrobial peptide: the proline hinge is responsible for the cell-penetrating ability of buforin II. Proc. Natl. Acad. Sci. 97: 8245–8250 (2000).
Acknowledgments
This work was funded by the Malaysia Ministry of Science, Technology and Innovation (MOSTI) under Project No. 10-05ABI-FB 037.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ghanbari, R., Ebrahimpour, A. Separation and identification of bromelain-generated antibacterial peptides from Actinopyga lecanora . Food Sci Biotechnol 27, 591–598 (2018). https://doi.org/10.1007/s10068-017-0267-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10068-017-0267-z