Abstract
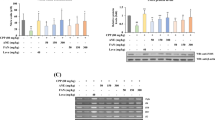
Polysaccharide (SJP) was extracted from Sea cucumber, Stichopus japonicas, and its immune-enhancing activities were evaluated in vivo immune-suppressed mice systems. Cyclophosphamide(CY)-treated mice were orally administrated with SJP according to different concentrations. The results showed that administration of SJP significantly increased spleen index without variation of the body weight, compared to only CY treatment group. The proliferation of splenic lymphocyte and NK activity was also stimulated by SJP. In addition, the oral administration of SJP up-regulated COX-2 and TLR-4 as well as cytokines such as IL-1β, IL-4, IL-6, IL-10, TNF-α and IFN-γ, which are secreted from splenic lymphocytes in cyclophosphamide-treated mice. Moreover, our results showed that SJP stimulated macrophages via NF-κB and MAPK signaling pathways. These findings provided the potential use of SJP as an alternative means under immune-suppressed conditions, and furthermore can be utilized as a functional material for food and pharmaceutical industries.





Similar content being viewed by others
References
Walls J, Sinclair L, Finlay D. Nutrient sensing, signal transduction and immune responses. Semin Immunol. 28: 396–407 (2016)
Miccadei S, Masella R, Mileo AM, Gessani S. Omega3 polyunsaturated fatty acids as immunomodulators in colorectal cancer: New potential role in adjuvant therapies. Front Immunol. 7: 486 (2016)
Jesenak M, Urbancikova I, Banovcin P. Respiratory tract infections and the role of biologically active polysaccharides in their management and prevention. Nutrients. 9: 779–790 (2017)
Liu X, Lin Q, Yan Y, Peng F, Sung R, Ren J. Hemicellulose from plant biomass in medical and pharmaceutical application: A critical review. Curr Med Chem. (2017). https://doi.org/10.2174/0929867324666170705113657
Patel S, Goyal A. Chitin and chitinase: Role in pathogenicity, allergenicity and health. Int J Biol Macromol. 97: 331–338 (2017)
Daien CI, Pinget GV, Tan JK, Macia L. Detrimental impact of microbiota-accessible carbohydrate-deprived diet on gut and immune homeostasis: An overview. Front Immunol. 8: 548 (2017)
Bordbar S, Anwar F, Saari N. High-value components and bioactives from sea cucumbers for functional foods—a review. Mar Drugs. 9: 1761–1805 (2011)
Cao RA, Surayot U, You S. Structural characterization of immunostimulating protein-sulfated fucan complex extracted from the body wall of a sea cucumber, Stichopus japonicus. Int J Biol Macromol. 99: 539–548 (2017)
Fredalina BD, Ridzwan BH, Abidin AA, Kaswandi MA, Zaiton H, Zali I, Kittakoop P, Jais AM. Fatty acid compositions in local sea cucumber, Stichopus chloronotus, for wound healing. Gen Pharmacol. 33: 337–340 (1999)
Liu X, Sun Z, Zhang M, Meng X, Xia X, Yuan W, Xue F, Liu C. Antioxidant and antihyperlipidemic activities of polysaccharides from sea cucumber Apostichopus japonicus. Carbohydr Polym. 90: 1664–1670 (2012)
Ustyuzhanina NE, Bilan MI, Dmitrenok AS, Shashkov AS, Kusaykin MI, Stonik VA, Nifantiev NE, Usov AI. Structure and biological activity of a fucosylated chondroitin sulfate from the sea cucumber Cucumaria japonica. Carbohydr Polym. 26: 449–459 (2016)
Cao RA, Lee SH, You SG. Structural effects of sulfated-glycoproteins from Stichopus japonicus on the nitric oxide secretion ability of RAW 264.7 cells. Prev Nutr Food Sci. 19: 307–313 (2014)
Sevag MG, Lackman DB, Smolens J. The isolation of the components of streptococcal nucleoproteins in serologically active form. J Biol Chem. 124: 425–436 (1938)
Ray A, Dittel BN. Isolation of mouse peritoneal cavity cells. J Vis Exp. 35: 1488 (2010)
Kim JK, Cho ML, Karnjanapratum S, Shin IS, You SG. In vitro and in vivo immunomodulatory activity of sulfated polysaccharides from Enteromorpha prolifera. Int J Biol Macromol. 49: 1051–1058 (2011)
Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR. Analysis of nitrate, nitrite, and [15N] nitrate in biological fluids. Anal Biochem. 126: 131–138 (1982)
Wang J, Tong X, Li P, Cao H, Su W. Immuno-enhancement effects of Shenqi Fuzheng Injection on cyclophosphamide-induced immunosuppression in Balb/c mice. J Ethnopharmacol. 139: 788–795 (2012)
Weeks BA, Keisler AS, Myrvik QN, Warinner JE. Differential uptake of neutral red by macrophages from three species of estuarine fish. Dev Comp Immunol. 11: 117–124 (1987)
Cho CW, Han CJ, Rhee YK, Lee YC, Shin KS, Shin JS, Lee KT, Hong HD. Cheonggukjang polysaccharides enhance immune activities and prevent cyclophosphamide-induced immunosuppression. Int J Biol Macromol. 72: 519–525 (2015)
Park HR, Lee HS, Cho SY, Kim YS, Shin KS. Anti-metastatic effect of polysaccharide isolated from Colocasia esculenta is exerted through immunostimulation. Int J Mol Med. 31: 361–368 (2013)
Sarangi I, Ghosh D, Bhutia SK, Mallick SK, Maiti TK. Anti-tumor and immunomodulating effects of Pleurotus ostreatus mycelia-derived proteoglycans. Int Immunopharmacol. 6: 1287–1297 (2006)
Narayanan BA, Narayanan NK, Simi B, Reddy BS. Modulation of inducible nitric oxide synthase and related proinflammatory genes by the omega-3 fatty acid docosahexaenoic acid in human colon cancer cells. Cancer Res. 63: 972–979 (2003)
Bhattacharyya S, Ratajczak CK, Vogt SK, Kelley C, Colonna M, Schreiber RD, Muglia LJ. TAK1 targeting by glucocorticoids determines JNK and IkappaB regulation in Toll-like receptor-stimulated macrophages. Blood. 115: 1921–1931 (2010)
Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 25: 402–408 (2001)
Yun KJ, Kim JY, Kim JB, Lee KW, Jeong SY, Park HJ, Jung HJ, Cho YW, Yun K, Lee KT. Inhibition of LPS-induced NO and PGE2 production by asiatic acid via NF-kappa B inactivation in RAW 264.7 macrophages: possible involvement of the IKK and MAPK pathways. Int Immunopharmacol. 8: 431–441 (2008)
Underhill DM, Ozinsky A, Hajjar AM, Stevens A, Wilson CB, Bassetti M, Aderem A. The Toll-like receptor 2 is recruited to macrophage phagosomes and discriminates between pathogens. Nature. 401: 811–815 (1999)
Bartl MM, Luckenbach T, Bergner O, Ullrich O, Koch-Brandt C. Multiple receptors mediate apoJ-dependent clearance of cellular debris into nonprofessional phagocytes. Exp Cell Res. 271: 130–141 (2001)
Navegantes KC, de Souza Gomes R, Pereira PAT, Czaikoski PG, Azevedo CHM, Monteiro MC. Immune modulation of some autoimmune diseases: the critical role of macrophages and neutrophils in the innate and adaptive immunity. J Transl Med. 15: 36 (2017)
Lori A, Perrotta M, Lembo G, Carnevale D. The spleen: A hub connecting nervous and immune systems in cardiovascular and metabolic diseases. Int J Mol Sci. 18: 1216–1227 (2017)
Chen X, Nie W, Fan S, Zhang J, Wang Y, Lu J, Jin L. A polysaccharide from Sargassum fusiforme protects against immunosuppression in cyclophosphamide-treated mice. Carbohydr Polym. 90: 1114–1119 (2012)
Sinkora M, Butler JE. Progress in the use of swine in developmental immunology of B and T lymphocytes. Dev Comp Immunol. 58: 1–17 (2016)
O’Sullivan TE, Sun JC, Lanier LL. Natural Killer Cell Memory. Immunity. 43: 634–645 (2015)
Turner MD, Nedjai B, Hurst T, Pennington DJ. Cytokines and chemokines: At the crossroads of cell signalling and inflammatory disease. Biochim Biophys Acta. 1843: 2563–2582 (2014)
Karavitis J, Hix LM, Shi YH, Schultz RF, Khazaie K, Zhang M. Regulation of COX2 expression in mouse mammary tumor cells controls bone metastasis and PGE2-induction of regulatory T cell migration. PLoS One. 7: e46342 (2012)
Reynolds JM, Martinez GJ, Chung Y, Dong C. Toll-like receptor 4 signaling in T cells promotes autoimmune inflammation. Proc Natl Acad Sci U S A. 109: 13064–13069 (2012)
Raphael I, Nalawade S, Eagar TN, Forsthuber TG. T cell subsets and their signature cytokines in autoimmune and inflammatory diseases. Cytokine. 74: 5–17 (2015)
Tak PP, Firestein GS. NF-kappaB: a key role in inflammatory diseases. J Clin Invest. 107: 7–11 (2001)
Kim JB, Han AR, Park EY, Kim JY, Cho W, Lee J, Seo EK, Lee KT. Inhibition of LPS-induced iNOS, COX-2 and cytokines expression by poncirin through the NF-kappaB inactivation in RAW 264.7 macrophage cells. Biol Pharm Bull. 30: 2345–2351 (2007)
Cargnello M, Roux PP. Activation and function of the MAPKs and their substrates, the MAPK-activated protein kinases. Microbiol Mol Biol Rev. 75: 50–83 (2011)
Acknowledgements
This study was supported by the Marine Bio-Regional Specialization Leading Technology Development Program (D11413914H480000100) funded by the Ministry of Oceans and Fisheries in Korea.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Monmai, C., Park, S.H., You, S. et al. Immuno-enhancement effect of polysaccharide extracted from Stichopus japonicus on cyclophosphamide-induced immunosuppression mice. Food Sci Biotechnol 27, 565–573 (2018). https://doi.org/10.1007/s10068-017-0248-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10068-017-0248-2