Abstract
Objective
To investigate the relationship between leptin, osteopontin (OPN), sclerostin (SOST) and severity of knee osteoarthritis (KOA).
Methods
The study included 148 consecutive patients with knee OA and 101 non-KOA subjects enrolled in this cross-sectional study. All patients fulfilled the American College of Rheumatology criteria for primary knee OA. Severity of the disease was assessed using plain radiography of the affected knee, according to the Kellgren and Lawrence classification. Fasting blood samples were obtained from all patients and controls; the serum samples were kept at − 80 °C before assessment of leptin, OPN, and SOST using a multiplex particle-based flow cytometric assay.
Results
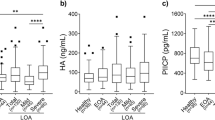
KOA patients group compared with the control group, serum leptin (KOA, 26581.7 ± 2011.5 pg/ml, vs control,6936.4 ± 702.2 pg/ml),OPN (KOA, 4908.3 ± 769.4 pg/ml, vs control, 2182.5 ± 217.8 pg/ml), and SOST (KOA, 2481.9 ± 543.5 pg/ml, vs control, 1288.9 ± 267.7 pg/ml) in the KOA group were higher than control group; there were also differences in three bone metabolic factors between male and female in the KOA group; meanwhile, there was correlation between each factor and the incidence of KOA.
Conclusion
Our study of 249 serum samples was conducted. Serum leptin, OPN, and SOST were significantly increased in KOA patients, and there was an internal correlation; these findings could, at best, contribute to the identification of novel targets for medical interventions.
Key Points • The aim of this study was to assess the relationships of radiographic knee OA with altered serum levels of leptin, OPN, and SOST. Our study of 249 serum samples was conducted. Serum leptin, OPN, and SOST were significantly increased in KOA patients compared with control group. There were gender differences in the concentration of three serum bone turnover factors in KOA group and control group. Serum SOST concentration increased with Kellgren-Lawrence (K-L) grading. We found that serum leptin, OPN, and SOST were significantly increased in KOA patients, and there was an internal correlation. Leptin had a remarkable diagnostic value in the incidence of KOA. |

Similar content being viewed by others
Abbreviations
- OA:
-
Osteoarthritis
- KOA:
-
knee osteoarthritis
- OPN:
-
osteopontin
- SOST:
-
sclerostin:
- K-L:
-
Kellgren-Lawrence
- BMI:
-
body mass index
References
Felson DT, Dsc YZ, Hannan MT, Naimark A, Levy D (1995) The incidence and natural history of knee osteoarthritis in the elderly. The Framingham osteoarthritis study. Arthritis Rheum 38(10):1500–1505
Leung GJ, Rainsford KD, Kean WF (2014) Osteoarthritis of the hand I: aetiology and pathogenesis, risk factors, investigation and diagnosis. J Pharm Pharmacol 66(3):339–346
Garnero P (2001) Cross sectional evaluation of biochemical markers of bone, cartilage, and synovial tissue metabolism in patients with knee osteoarthritis: relations with disease activity and joint damage. Ann Rheum Dis 60(6):619–626
Atkins GJ, Rowe PS, Lim HP, Welldon KJ, Ormsby R, Wijenayaka AR et al (2011) Sclerostin is a locally acting regulator of late-osteoblast/preosteocyte differentiation and regulates mineralization through a mepe-asarm-dependent mechanism. J Bone Miner Res 26(7)
Blom AB, Peter LVL, Peter MVDK, Wim BVDB (2010) To seek shelter from the wnt in osteoarthritis? Wnt-signaling as a target for osteoarthritis therapy. Curr Drug Targets 11(5):620–629
Bouaziz W, Funck-Brentano T, Lin H, Marty C, Ea HK, Hay E, Cohen-Solal M (2015) Loss of sclerostin promotes osteoarthritis in mice via β-catenin-dependent and -independent wnt pathways. Arthritis Res Ther 17(1):24
Mabey T, Honsawek S, Tanavalee A, Wilairatana V, Yuktanandana P, Saetan N, Zhan D (2014) Plasma and synovial fluid sclerostin are inversely associated with radiographic severity of knee osteoarthritis. Clin Biochem 47(7–8):547–551. https://doi.org/10.1016/j.clinbiochem.2014.03.011
Denhardt DT, Noda M (1998) Osteopontin expression and function: role in bone remodeling. 72(Supplement 30):92–102
Attur MG, Dave MN, Stuchin S, Kowalski AJ, Steiner G, Abramson SB, Denhardt DT, Amin AR (2001) Osteopontin: an intrinsic inhibitor of inflammation in cartilage. Arthritis Rheum 44(3):578–584
Pullig O, Weseloh G, Gauer S, Swoboda B (2000) Osteopontin is expressed by adult human osteoarthritic chondrocytes: protein and mRNA analysis of normal and osteoarthritic cartilage. Matrix Biol J Int Soc Matrix Biol 19(3):245–255
Green ED, Maffei M, Braden VV, Proenca R, Desilva U, Zhang Y et al (1995) The human obese (Ob) gene: RNA expression pattern and mapping on the physical, cytogenetic, and genetic maps of chromosome 7. Genome Res 5(1):5–12
Cowley MA, Smart JL, Rubinstein M, Cerdan MG, Diano S, Horvath TL et al (2001) Leptin activates anorexigenic pomc neurons through a neural network in the arcuate nucleus. Nature 411(6836):480–484
Liang J, Feng J, Wu WKK, Xiao J, Wu Z, Han D, Zhu Y, Qiu G (2011) Leptin-mediated cytoskeletal remodeling in chondrocytes occurs via the rhoa/rock pathway. J Orthop Res 29(3):369–374
Scotece M, Conde J, López V, Lago F, Pino J, Gómez-Reino JJ, Gualillo O (2014) Adiponectin and leptin: new targets in inflammation. Basic Clin Pharmacol Toxicol 114(1):97–102
Altman RD, Asch E, Bloch DA, Bole G, Hochberg M (1986) Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Diagnostic and therapeutic criteria committee of the American rheumatism association. Arthritis Rheum 29(8):1039–1049
Kellgren JH, Lawrence JS (1957) Radiological assessment of osteoarthrosis. Ann Rheum Dis 16(4):494–502
Karvonen-Gutierrez CA, Harlow SD, Mancuso P, Jacobson J, Mendes de Leon CF, Nan B (2013) Association of leptin levels with radiographic knee osteoarthritis among a cohort of midlife women. Arthritis Care Res 65(6):936–944
Ahima RS, Prabakaran D, Mantzoros C, Qu D, Lowell B, Maratos-Flier E, Flier JS (1996) Role of leptin in the neuroendocrine response to fasting. Nature (London) 382(6588):250–252
Chang IC, Chiang TI, Yeh KT, Lee H, Cheng YW (2010) Increased serum osteopontin is a risk factor for osteoporosis in menopausal women. Osteoporos Int 21(8):1401–1409
Ardawi M-SM, Al-Kadi HA, Rouzi AA et al (2011) Determinants of serum sclerostin in healthy pre- and postmenopausal women. J Bone Miner Res 26(12)
Cicuttini FM, Baker JR, Spector TD (1996) The association of obesity with osteoarthritis of the hand and knee in women: a twin study. J Rheumatol 23:1221–1226
Iliopoulos D, Malizos KN, Tsezou A (2007) Epigenetic regulation of leptin affects mmp-13 expression in osteoarthritic chondrocytes: possible molecular target for osteoarthritis therapeutic intervention. Ann Rheum Dis 66(12):1616–1621
Masuzaki H, Ogawa Y, Isse N, Satoh N, Okazaki T, Shigemoto M, Mori K, Tamura N, Hosoda K, Yoshimasa Y, Jingami H, Kawada T, Nakao K (1995) Human obese gene expression: adipocyte-specific expression and regional differences in the adipose tissue. Diabetes 44(7):855–858
Pelleymounter MA, Cullen MJ, Baker MB, Hecht R, Winters D, Boone T (1995) Effects of the obese gene product on body weight regulation in Ob/Ob mice. Science 269(5223):540–543
Zhang P, Zhong ZH, Yu HT, Liu B (2015) Significance of increased leptin expression in osteoarthritis patients. PLoS One:10
Dumond H, Presle N, Terlain B, Mainard D, Loeuille D, Netter P et al (2014) Evidence for a key role of leptin in osteoarthritis. Arthritis Rheum 48(11):3118–3129
Scotece M, Mobasheri A et al (2015) Leptin in osteoarthritis: focus on articular cartilage and chondrocytes. Life Sci 140:S0024320515003161
Yang Y, Gao SG, Zhang FJ, Luo W, Lei GH (2014) Effects of osteopontin on the expression of il-6 and il-8 inflammatory factors in human knee osteoarthritis chondrocytes. Cell Biochem Biophys
Yusheng L, Wenfeng X, Minghua S, Zhenhan D, Chao Z, Hui L et al (2016) The expression of osteopontin and wnt5a in articular cartilage of patients with knee osteoarthritis and its correlation with disease severity. Biomed Res Int 2016:1–7
Mohammed FI, Abd El-Azeem MI, Kamaleldin AM (2012) Plasma and synovial fluid osteopontin levels in patients with knee osteoarthritis: relation to radiological grade. Egypt Rheumatol 34(3):131–136
Honsawek S, Tanavalee A, Sakdinakiattikoon M, Chayanupatkul M, Yuktanandana P (2009) Correlation of plasma and synovial fluid osteopontin with disease severity in knee osteoarthritis. Clin Biochem 42(9):808–812
Gao SG, Li KH, Zeng KB, Tu M, Xu M, Lei GH (2010) Elevated osteopontin level of synovial fluid and articular cartilage is associated with disease severity in knee osteoarthritis patients. Osteoarthr Cartil 18(1):82–87
Mödder UI, Hoey KA, Amin S, Mccready LK, Achenbach SJ, Riggs BL et al (2011) Relation of age, gender, and bone mass to circulating sclerostin levels in women and men. J Bone Miner Res 26(2):373–379
Semenov M, Tamai K, He X (2005) SOST is a ligand for lrp5/lrp6 and a wnt signaling inhibitor. J Biol Chem 280(29):26770–26775
Moustafa A, Sugiyama T, Prasad J, Zaman G, Gross TS, Lanyon LE, Price JS (2012) Mechanical loading-related changes in osteocyte sclerostin expression in mice are more closely associated with the subsequent osteogenic response than the peak strains engendered. Osteoporos Int 23(4):1225–1234
Robling AGA, Niziolek PJP, Baldridge LAL, Condon KWK, Allen MRM, Alam II et al (2008) Mechanical stimulation of bone in vivo reduces osteocyte expression of SOST/sclerostin. J Biol Chem 283(9):5866–5875
Wanby P, Nobin R, Von SP, Brudin L, Carlsson M (2016) Serum levels of the bone turnover markers dickkopf-1, sclerostin, osteoprotegerin, osteopontin, osteocalcin and 25-hydroxyvitamin d in swedish geriatric patients aged 75?Years or older with a fresh hip fracture and in healthy controls. J Endocrinol Investig 39:855–863
Funding
This work was supported by the National Science Foundation of China (81672239).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Disclosures
None.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the regional research committee (Regional Ethical Review Board for CFDA-GCP/ICH-GCP) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Min, S., Shi, T., Han, X. et al. Serum levels of leptin, osteopontin, and sclerostin in patients with and without knee osteoarthritis. Clin Rheumatol 40, 287–294 (2021). https://doi.org/10.1007/s10067-020-05150-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-020-05150-z